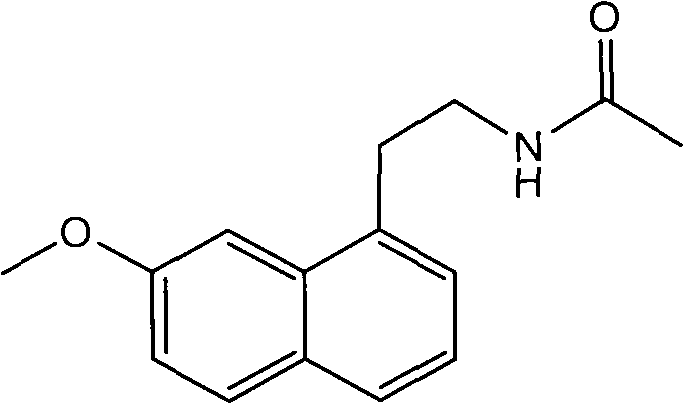
Agomelatine tablet and preparation method thereof, as well as coated tablet of agomelatine tablet and preparation method thereof
A technology for agomelatine tablets and coated tablets, which is applied in the field of agomelatine tablets and its preparation, and can solve the problems of unfavorable drug stable release, unfavorable tablet storage, and fast dissolution of tablets, etc. Achieve the effects of strong industrial feasibility, reasonable selection, and stable preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
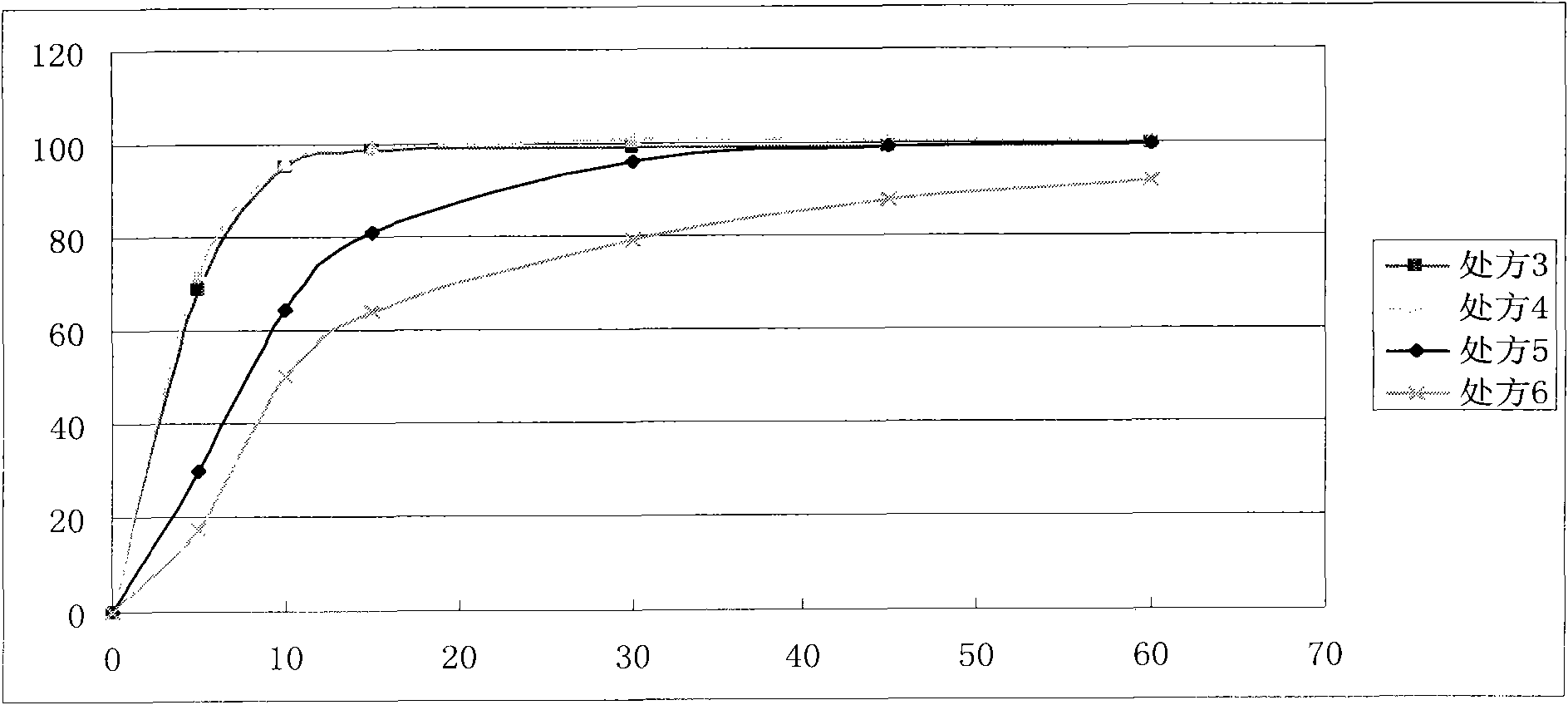
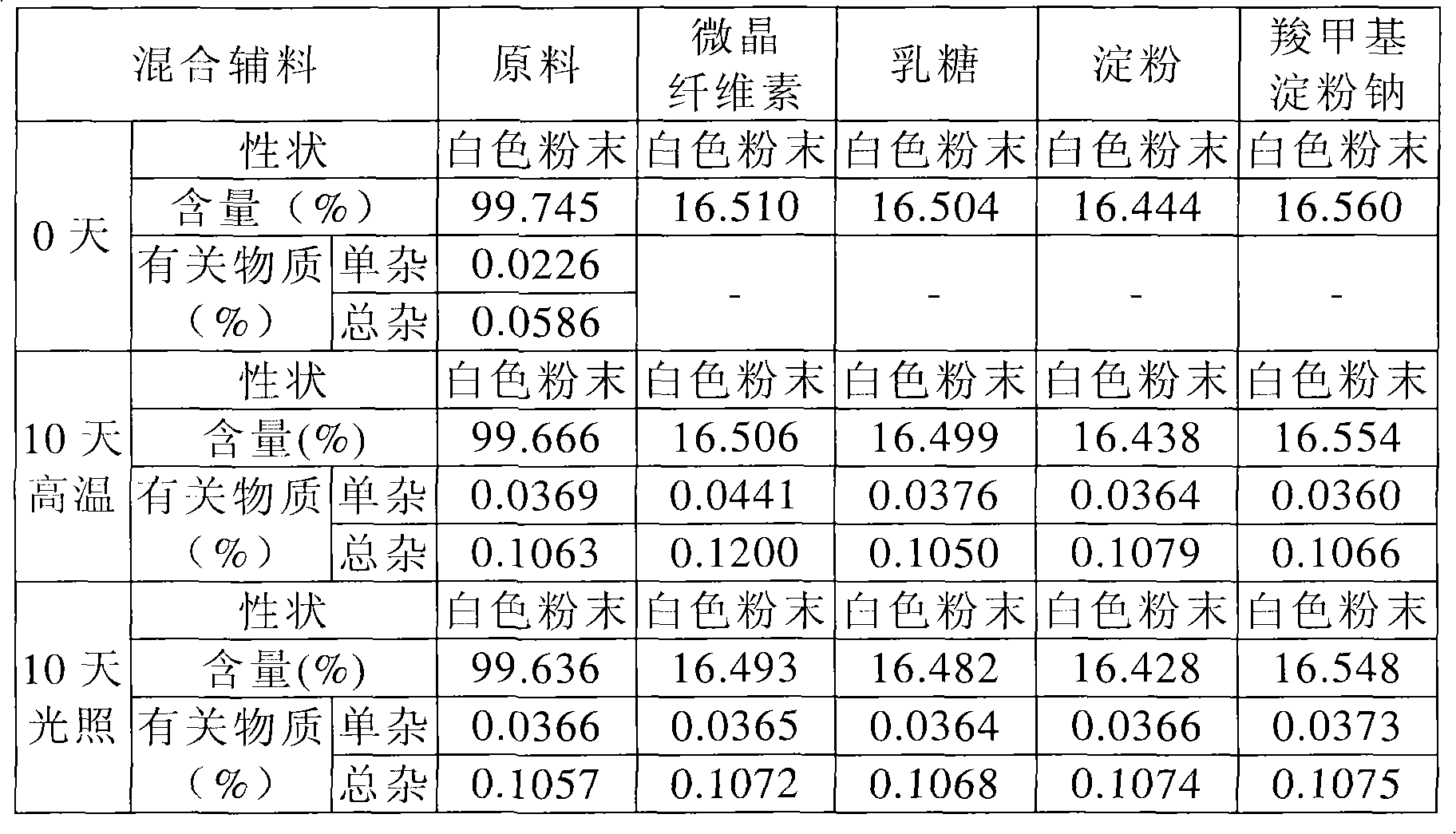
Image
Examples
preparation example Construction
[0056] The preparation method of agomelatine tablet of the present invention is preferably:
[0057] (1) The raw and auxiliary materials are crushed and sieved for later use;
[0058] (2) Povidone K30 is weighed to be mixed with a 10% by weight aqueous solution;
[0059] (3) Accurately weigh each raw and auxiliary material according to the prescription amount, mix agomelatine with lactose, starch, and sodium carboxymethyl starch, add an appropriate amount of 10% by weight of povidone K30 aqueous solution, and make a soft material;
[0060] (4) Soft materials are sieved to prepare wet granules and dried;
[0061] (5) granulate, add prescription amount magnesium stearate, silicon dioxide, mix evenly;
[0062] (6) Determination of main drug content, calculation of tablet weight;
[0063] (7) Tablets.
[0064] The standards and definitions of suitable soft materials can be defined in accordance with the 6th edition of "Pharmacy" published by People's Medical Publishing House i...
Embodiment 1
[0139] Embodiment 1: agomelatine tablet
[0140] prescription:
[0141] Raw material name Prescription dosage (g)
[0142] agomelatine 25
[0143] Lactose 70
[0144] starch 98
[0145] Sodium carboxymethyl starch 1
[0146] povidone k30 2
[0147] Silica 2
[0149] Makes 1000 pieces
[0150] Preparation:
[0151] (1) The raw and auxiliary materials are respectively pulverized and passed through a 100-mesh sieve for subsequent use;
[0152] (2) Take povidone K30, and be mixed with 10% by weight aqueous solution;
[0153] (3) Accurately weigh each raw and auxiliary material according to the prescription amount, mix agomelatine with lactose, starch, and sodium carboxymethyl starch, add an appropriate amount of 10% by weight of povidone K30 aqueous solution, and make a soft material;
[0154] (4) Soft materials are passed through a 20-mesh sieve to prepare wet granules, and dried at 60°C until the moisture content of the granules is 2-4%; ...
Embodiment 2
[0158] Embodiment 2: agomelatine film-coated tablet
[0159] Tablet prescription:
[0160] Raw material name Prescription dosage (g)
[0161] agomelatine 25
[0162] Lactose 70
[0163] starch 98
[0164] Sodium carboxymethyl starch 1
[0165] povidone k30 2
[0166] Silica 2
[0168] Makes 1000 pieces
[0169] Coating Solution Prescription:
[0170] Opadry II 45g
[0171] Add purified water to 300g
[0172] 1000g tablet core dosage
[0173] The weight gain of the coating is 2-4%.
[0174] Preparation:
[0175] Tablet preparation:
[0176] (1) The raw and auxiliary materials are respectively pulverized and passed through a 100-mesh sieve for subsequent use;
[0177] (2) Take povidone K30, and be mixed with 10% by weight aqueous solution;
[0178] (3) Accurately weigh each raw and auxiliary material according to the prescription amount, mix agomelatine with lactose, starch, and sodium carboxymethyl starch, add an appro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com