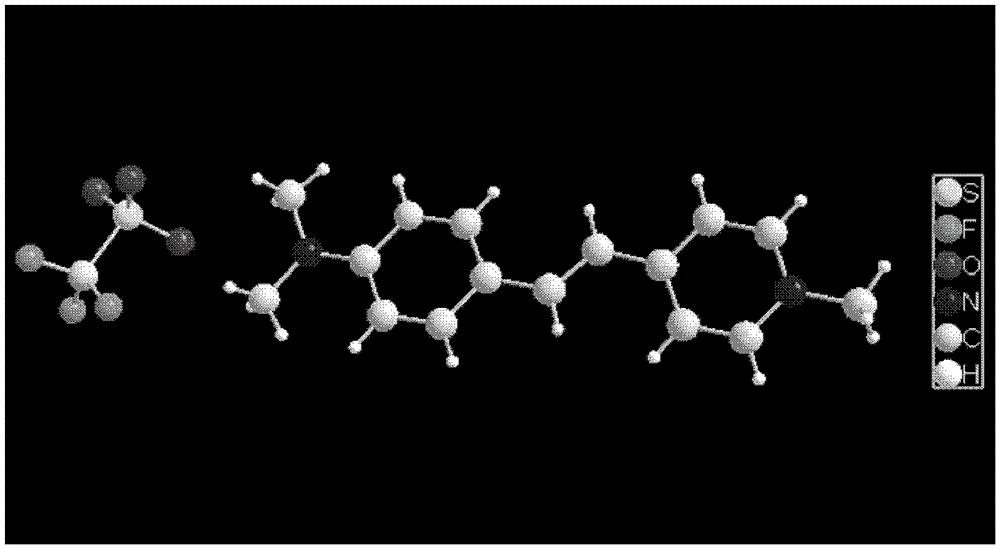
A kind of organic third-order nonlinear optical material 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate and its synthesis method
A technology of methylpyridine trifluorosulfonate and dimethylaminostyrene, applied in nonlinear optics, organic chemistry, sulfonate preparation, etc., can solve the problem of easy deliquescence, lattice distortion, and limit research progress and practical application and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 14-
[0020] The synthesis of implementation case 14-(4-dimethylaminostyryl) picoline iodide
[0021] Dissolve 0.01mol of 4-methylpyridine, 0.01mol of methyl iodide and 0.01mol of 4-dimethylaminobenzaldehyde in 100-150mL of methanol, stir and slowly heat up to 65-70°C, add 3-5mL of piperidine as a catalyst Reflux reaction for 10 to 12 hours, the color of the solution gradually changed from light yellow to deep red, and the solution was crystallized by cooling, filtered, and dried to obtain 4-(4-dimethylaminostyryl)picoline iodide with a yield of It was 84.2%.
[0022] According to Example 1: 0.02mol of 4-picoline, 0.02mol of methyl iodide and 0.02mol of 4-dimethylaminobenzaldehyde were dissolved in 100-150mL of methanol, stirred and slowly heated to 65-70°C and refluxed for 48h, The color of the solution gradually changed from light yellow to dark red. After the solution was crystallized by cooling, filtered and dried, 4-(4-dimethylaminostyryl)picoline iodide was obtained with a yi...
Embodiment example 24-
[0024] Implementation Case 24-(4-dimethylaminostyryl) synthesis of picoline trifluorosulfonate
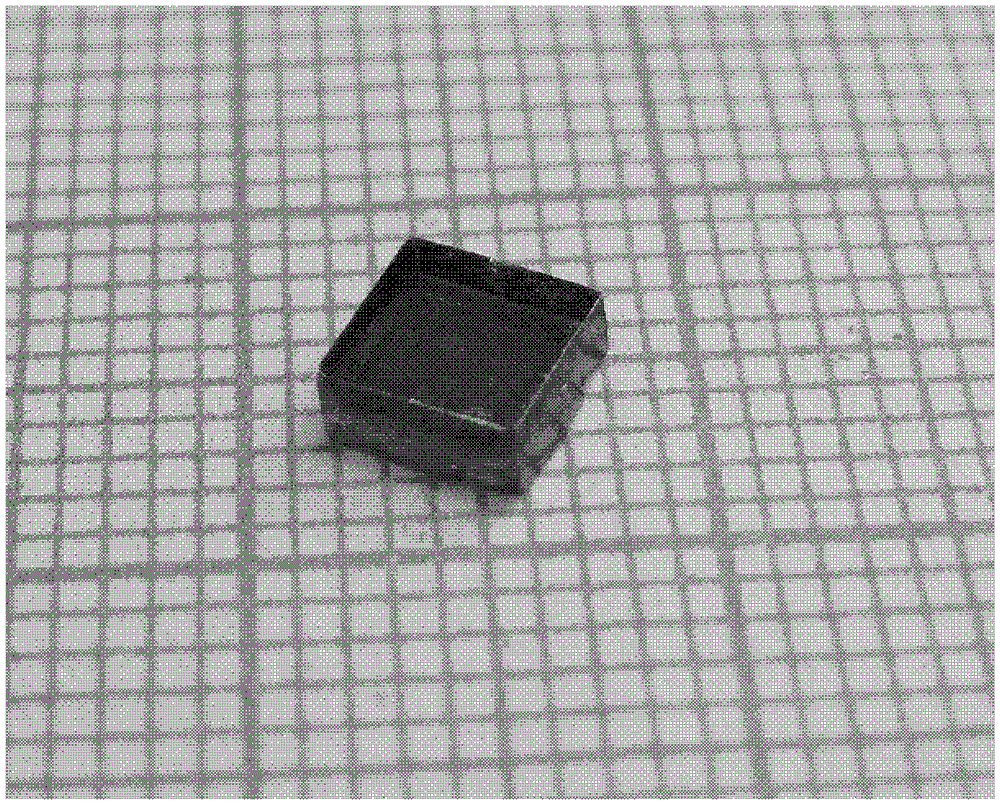
[0025] Under the condition of 50°C, react 0.01mol 4-(4-dimethylaminostyryl)picoline iodide with 0.01~0.012mol silver trifluorosulfonate in 50~75mL methanol for about 2h, filter to remove the precipitate, The solution was left to stand, cooled and crystallized to obtain red needle-like or massive 4-(4-dimethylaminostyryl)picoline trifluorosulfonate crystals, with a yield of 90.4%.
[0026] At room temperature, react 0.01mol 4-(4-dimethylaminostyryl)picoline iodide with 0.01~0.012mol silver trifluorosulfonate in 50~75mL methanol for about 2 hours, remove the precipitate by filtration, and pass the solution through static Place and slowly evaporate the solvent to obtain red blocky 4-(4-dimethylaminostyryl)picoline trifluorosulfonate crystals with a yield of 84.7%.
[0027] Under the condition of 50°C, 0.01mol of 4-(4-dimethylaminostyryl)picoline iodide was dissolved in 75mL of methan...
Embodiment example 34-
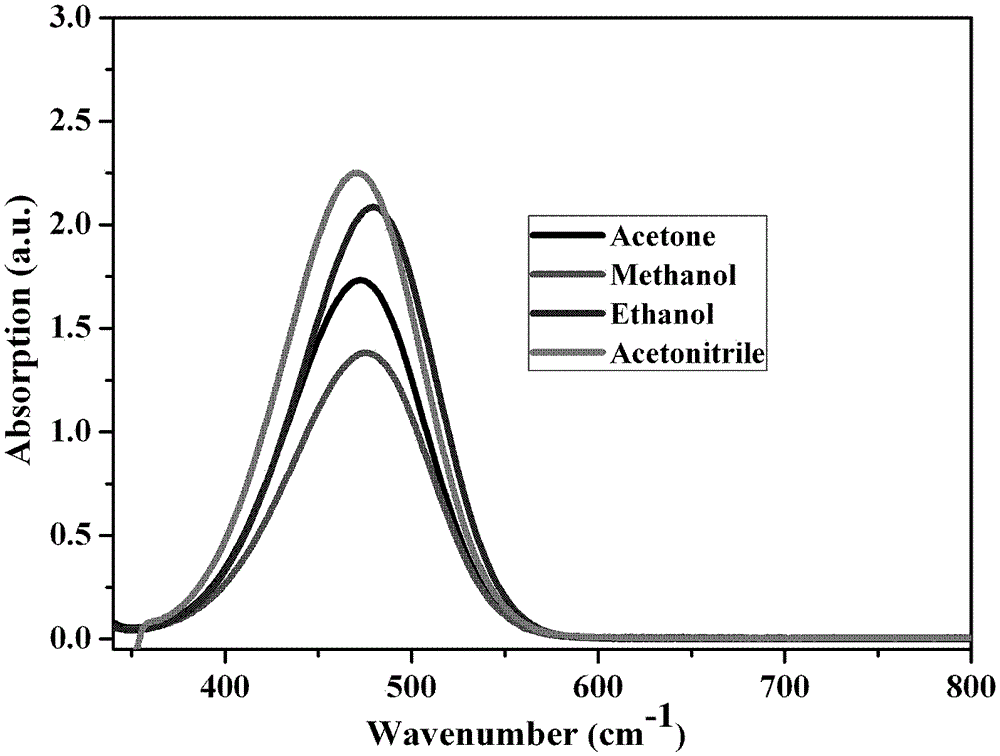
[0028] Implementation Case 3 Test of third-order nonlinear optical properties of 4-(4-dimethylaminostyryl)picoline trifluorosulfonate
[0029] (1) Absorption spectrum of 4-(4-dimethylaminostyryl)picoline trifluorosulfonate
[0030] The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was formulated into 2×10 -5 mol / L acetone solution, measure its absorption spectrum in the range of 350-800nm.
[0031]The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was formulated into 2×10 -5 mol / L methanol solution, and measure its absorption spectrum in the range of 350-800nm.
[0032] The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was formulated into 2×10 -5 mol / L ethanol solution, and measure its absorption spectrum in the range of 350-800nm.
[0033] The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com