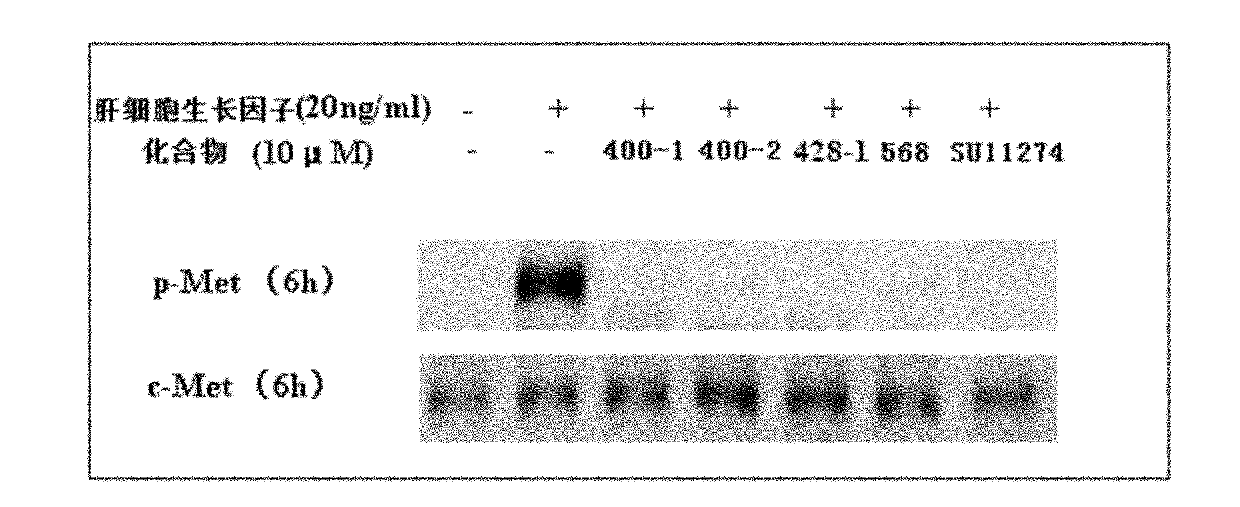
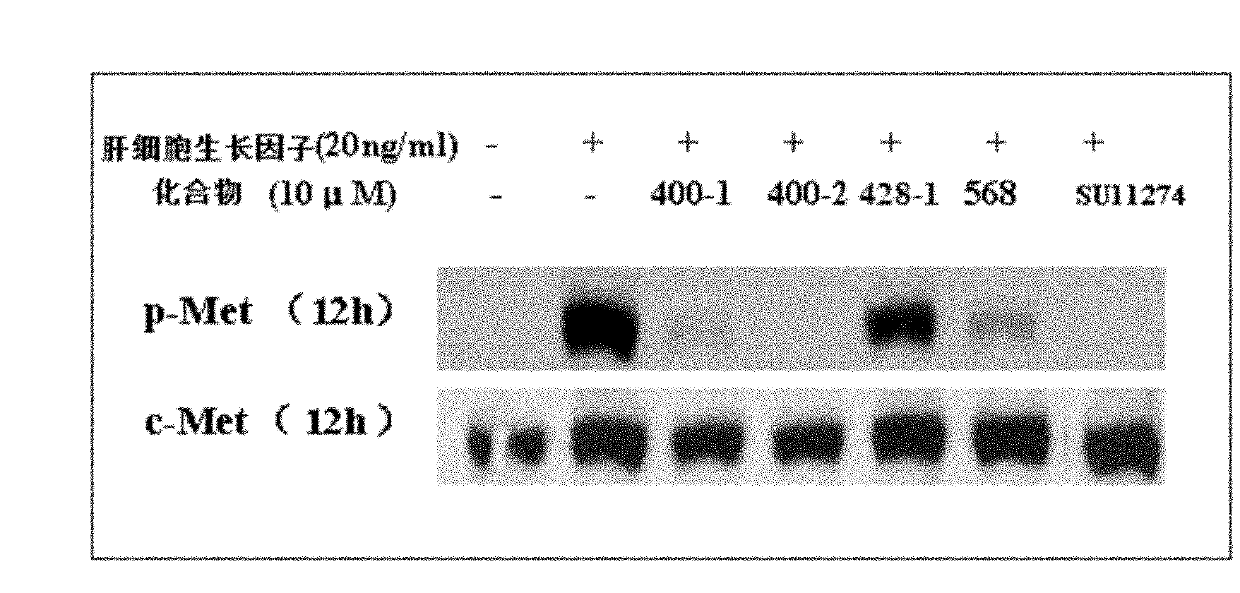
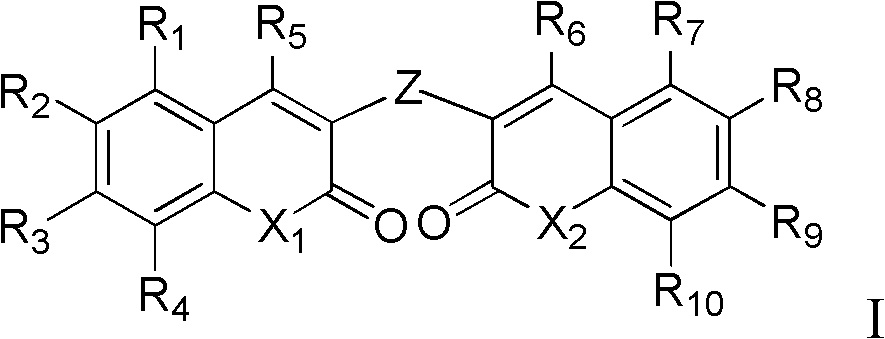
Dicoumarol compound, as well as preparation method and application thereof
A technology of dicoumarins and compounds, applied in the fields of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0049] Preparation Example 1 3,3'-methylenebis(4,7,8-trihydroxycoumarin) (compound number: 400-1)
[0050]
[0051]4,7,8-trihydroxycoumarin (preparation method: starting from 2,3,4 trihydroxy-acetophenone (commercially available), utilizing 2 equivalents of methoxymethyl chloride to protect the 3-position and 4-position The hydroxyl group is then reacted with NaH / diethyl carbonate in tetrahydrofuran (THF) to form a 4-hydroxyl substituted coumarin, and this intermediate can be deprotected by hydrochloric acid to obtain 4, 7, 8 - Trihydroxycoumarin) 500mg was dissolved in ethanol (10mL), added paraformaldehyde 50mg, and Et 3 N (0.5 mL) was reacted at room temperature for 24 h, filtered, washed with citric acid, and dried in vacuo to give off-white solid 400 (460 mg, 89%).
[0052] 1 H NMR (300MHz, DMSO-d6): 10.05(bs, 6H), 7.27(d, J=8.7Hz, 2H), 6.83(d, J=8.7Hz, 2H), 3.71(s, 2H).ESI- MS(m / z): 401.4(M+1) +
preparation Embodiment 2
[0053] Preparation Example 2 N-(7,8-dihydroxy-2(1H)-quinolinone-3-)-7,8-dihydroxyl-2(1H)-quinolinone-3-amide (compound number: 395-1)
[0054]
[0055] 7,8-bis(methoxymethoxy)-2(1H)quinolinone-3-acid (50mg, 0.16mmol) and 7,8-bis(methoxymethoxy)-2(1H) Quinolinone-3-amine (46 mg, 1 eq, 0.16 mmol) (prepared by reference method: Saari, Walfred S.; Wai, John S.; Fisher, Thorsten E.; Thomas, Craig M.; Hoffman, Jacob M. ; Etc. Journal of Medicinal Chemistry, 1992, vol.35, #21 p.3792-3802) was dissolved in 0.7mL DCM / 0.3mL pyridine solvent and then added EDCI, reacted at room temperature for 3h, the system changed from clear to turbid, filtered, washed with methanol , to obtain 55mg of solid compound, molar yield: 60%.
[0056] The above compound was dissolved in 3N HCl in ethyl acetate solution, and after adding 1d of methanol, the compound was obtained as a gray-green solid 38 mg. Molar yield: 100%.
[0057] 1 H NMR (300MHz, DMSO-d6): 12.42(s, 1H), 11.16(s, 1H), 10.87(s, 1H),...
preparation Embodiment 3
[0058] Preparation Example 3 7,7',8,8'-tetrahydroxy-4,4'-dimethyl-3,3'-dicoumarin (compound number: 382-1)
[0059]
[0060] 3-Bromo-4-methyl-7,8-bis(methoxymethoxy)-coumarin (100 mg, 0.278 mmol) (prepared according to literature methods: Bowden, Keith; Battah, Sinan Journal of the Chemical Society , Perkin Transactions 2: Physical Organic Chemistry (1972-1999), 1998, p.1603-1606), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (16mg, 0.05eq) and Cesium carbonate (135mg, 1.5eq, 0.417mmol) was dissolved in 10mL of anhydrous dioxane, degassed and added Pd(OAc) 2 (6mg, 0.05eq), raised to 100°C, 10% citric acid was carefully added after 12h to acidify, extracted, concentrated through column (DCM / MeOH=10 / 1), and 30mg compound was obtained. Molar yield: 39%. The above compound was dissolved in 3N HCl in ethyl acetate solution, and after adding 1 drop of methanol, 20 mg of the compound was obtained as a gray-green solid. Molar yield: 100%.
[0061] 1 H NMR (300MHz, CDCl 3 ): 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com