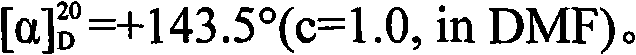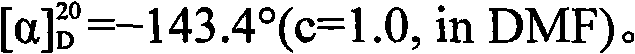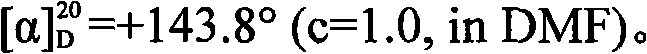Method for preparing optically pure cis apovincaminic acid ester
A vincristate, optical purity technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as separation and removal of product purification difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Preparation of D-cis-vincine methyl ester: at room temperature, add acetone 2500mL and racemic cis-vincine to a 5.0L three-necked bottle equipped with mechanical stirring, thermometer and spherical condenser 336.4 g (1.00 mol) of methyl ester, and slowly drop into it a solution of 394.1 g (1.10 mol) of L-dibenzoyl tartaric acid and 1000 mL of acetone, and stir while adding. After the addition, continue to control the temperature and stir the reaction, the solid gradually precipitates, and continue to stir for 2.0h. After filtering, the obtained crystals were washed with cold acetone (150mL×2), and dried under reduced pressure at 40-50°C for 3.0h to obtain 316.1g of dextro-apovincine methyl ester L-dibenzoyl tartrate, with a yield of 45.5 %, melting point 142°C (decomposition).
[0033] The obtained d-vincine methyl ester L-dibenzoyl tartrate was added to a mixture of 2500 mL of dichloromethane and 2000 mL of ice water, and the pH was adjusted to 9.0 with 25% ammonia wat...
Embodiment 2
[0037] Preparation of D-cis-vincine methyl ester: at room temperature, add acetonitrile 2000mL and racemic cis-vincine in turn to a 5.0L three-necked flask equipped with mechanical stirring, thermometer and spherical condenser 336.4 g (1.00 mol) of methyl ester, and slowly drop into it a solution of 394.1 g (1.10 mol) of L-dibenzoyl tartaric acid and 1000 mL of acetonitrile, stirring while adding. After the addition, continue to control the temperature and stir the reaction, the solid gradually precipitates, and continue to stir for 2.0h. After filtering, the obtained crystals were washed with cold acetonitrile (150mL×2), and dried under reduced pressure at 40-50°C for 3.0h to obtain 319.6g of dextro-apovincine methyl ester L-dibenzoyl tartrate, with a yield of 46.0 %, melting point 142°C (decomposition).
[0038] According to the method described in Example 1, 10% sodium carbonate solution was used instead of ammonia water to neutralize, release and crystallize the separated...
Embodiment 3
[0042] Preparation of D-cis-Apo-vincine methyl ester: At room temperature, add acetonitrile 1600mL, methanol 200mL and racemic cis-Apo Add 336.4 g (1.00 mol) of vinblastine methyl ester, and slowly drop into it a solution of 394.1 g (1.10 mol) of L-dibenzoyl tartaric acid and 1000 mL of acetonitrile, stirring while adding. After the addition, continue to control the temperature and stir the reaction, the solid gradually precipitates, and continue to stir for 2.0h. After filtration, the obtained crystals were washed with cold acetonitrile (150mL×2), and dried under reduced pressure at 40-50°C for 3.0h to obtain 284.8g of dextro-apovincine methyl ester L-dibenzoyl tartrate, with a yield of 41.0 %, melting point 142°C (decomposition).
[0043] According to the method described in Example 1, 10% potassium carbonate solution was used instead of ammonia water to neutralize, release and crystallize the separated and purified D-apovincine methyl ester L-dibenzoyl tartrate. Obtain 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com