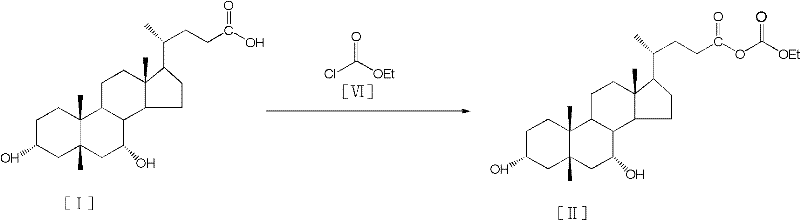
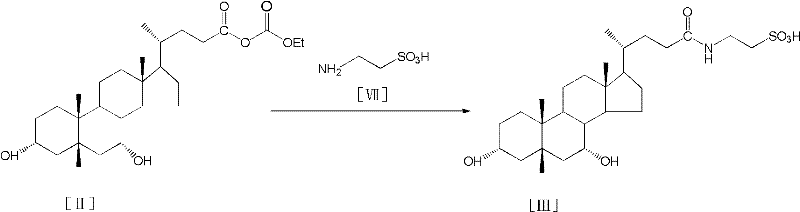
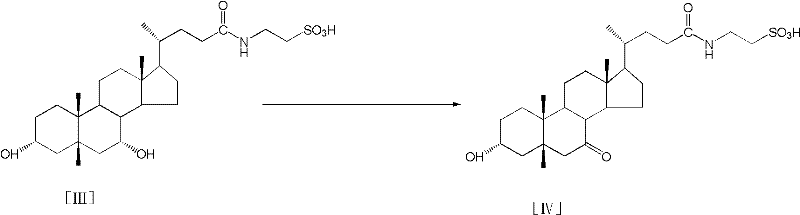
Process for preparing tauroursodeoxycholic acid hydrate
A technology of deoxycholic acid and taurine, which is applied in the direction of steroids and organic chemistry, can solve the problems of waste of resources and energy consumption, impact on product quality, long reaction process, etc., and achieve the goal of producing less three wastes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Add 25.0 g of chenodeoxycholic acid and 150 ml of acetone into a 1000 ml three-necked reaction flask, and stir evenly at 15°C; then add 8.9 ml of triethylamine, and stir at high speed for 60 min to make it into a solid suspension. Cool down to -10°C, add 6.5ml of ethyl chloroformate to react for 2 hours; after the reaction is completed, remove the insoluble matter by filtration to obtain the mother liquor of chenodeoxy mixed anhydride.
[0037] (2) 10.4g of sodium taurine, 0.5g of sodium hydroxide and 18.6ml of pure water were stirred and dissolved; after being cooled to room temperature, they were put into the obtained chenodeoxy mixed anhydride mother liquor of step (1), and stirred at a high speed at room temperature for 2 ~5h; after the reaction, adjust the pH value to 1~2, stir at a medium speed for 30min; slowly add 500ml of acetone, drop the temperature to 0~5°C after the dropwise addition, stir at a low speed for 2~5h; filter it, and wash it with a small amou...
Embodiment 2
[0041] 1) Add 25.0 g of chenodeoxycholic acid and 400 ml of dioxane to a 1000 ml three-necked reaction flask and stir at 15°C until uniform, then add 8.2 g of 4-dimethylaminopyridine and stir at high speed for 60 min. Cool down to -5°C, add 8.2ml of isobutyl chloroformate and react for 2h. After the reaction was completed, the insolubles were removed by filtration to obtain the mixed acid anhydride mother liquor.
[0042]2) 11.4g of potassium taurine, 0.5g of potassium hydroxide, and 18.6ml of pure water were stirred and dissolved, cooled to room temperature, and put into the mixed acid anhydride solution, and reacted with high-speed stirring at room temperature for 2 to 5 hours. After the reaction, adjust the pH value to 2-3, stir for 60 minutes, slowly add 500ml of dioxane, drop the temperature to 0-5°C and stir at a low speed for 2-5 hours, filter, wash with a small amount of frozen dioxane, and dry to obtain cattle 30.1 g of chenodeoxycholic acid sulfonate product.
[00...
Embodiment 3
[0046] 1) Add 25.0 g of chenodeoxycholic acid and 200 ml of cyclohexanone to a 1000 ml three-necked reaction flask and stir evenly at 15°C, then add 8.9 ml of pyridine and stir at high speed for 60 min to form a solid suspension. Cool down to -15°C, add 8.7ml of isobutyl chloroformate to react for 2h. After the reaction was completed, the insolubles were removed by filtration to obtain the mixed acid anhydride mother liquor.
[0047] 2) 10.3g of sodium taurine, 0.5g of barium hydroxide, and 18.6ml of pure water were stirred and dissolved, cooled to room temperature and then put into the mixed acid anhydride solution, and reacted with high-speed stirring at room temperature for 2 to 5 hours. After the reaction, adjust the pH value to 0.5-1, stir for 60 minutes, slowly add 600ml of cyclohexanone, drop the temperature to 0-5°C and stir at a low speed for 2-5 hours, filter, wash with a small amount of frozen cyclohexanone, and dry to obtain goose taurine Deoxycholic acid product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com