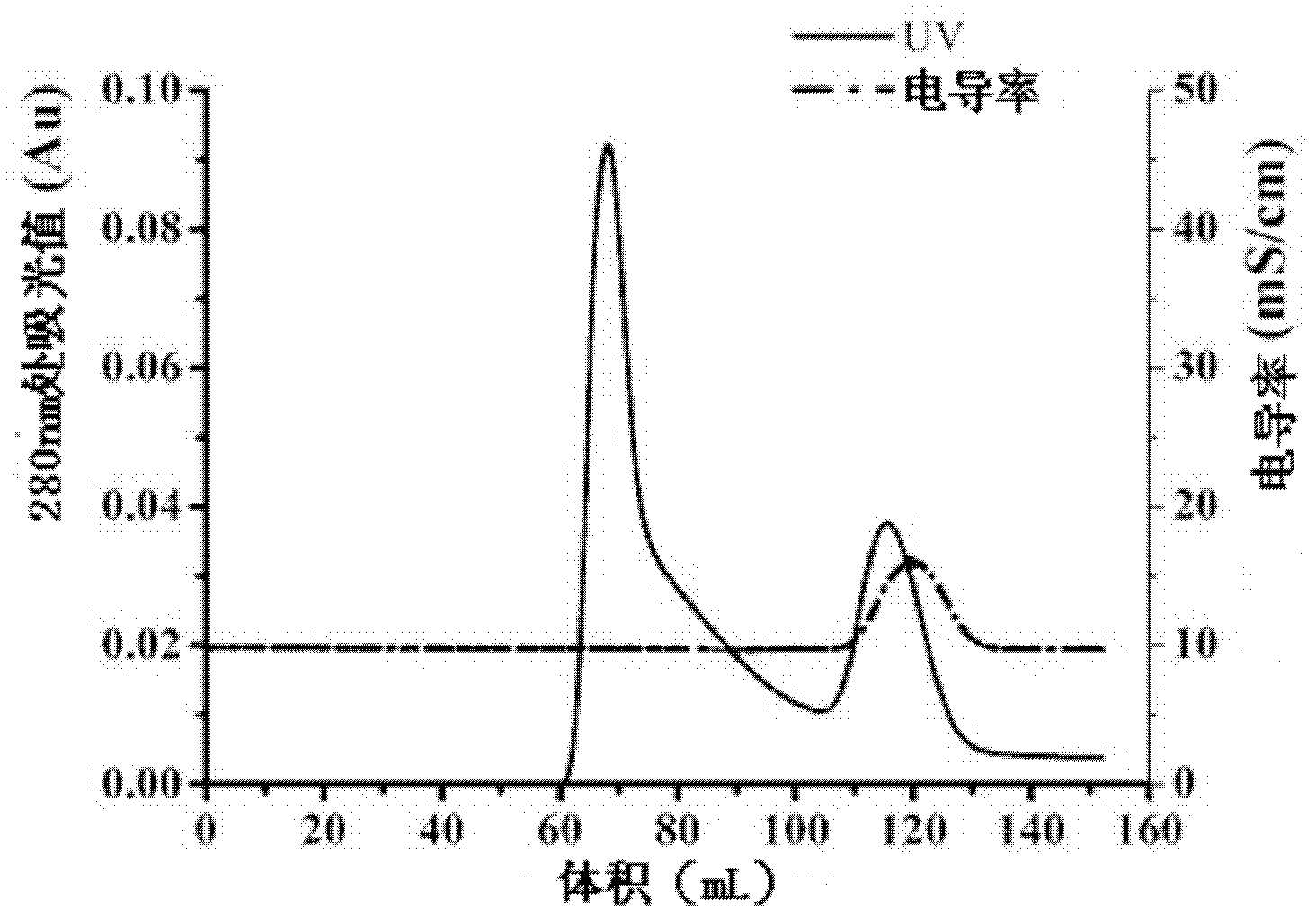
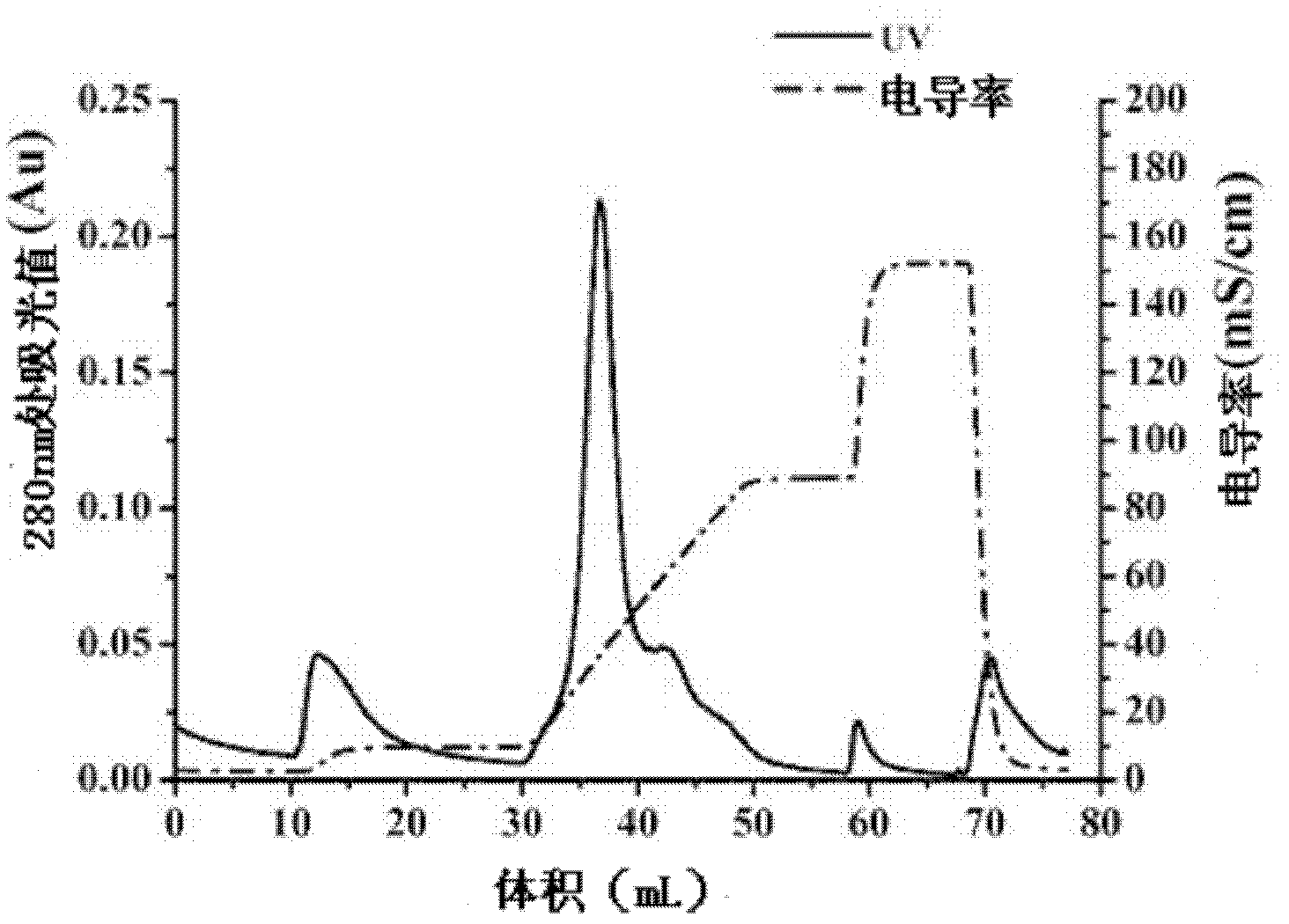
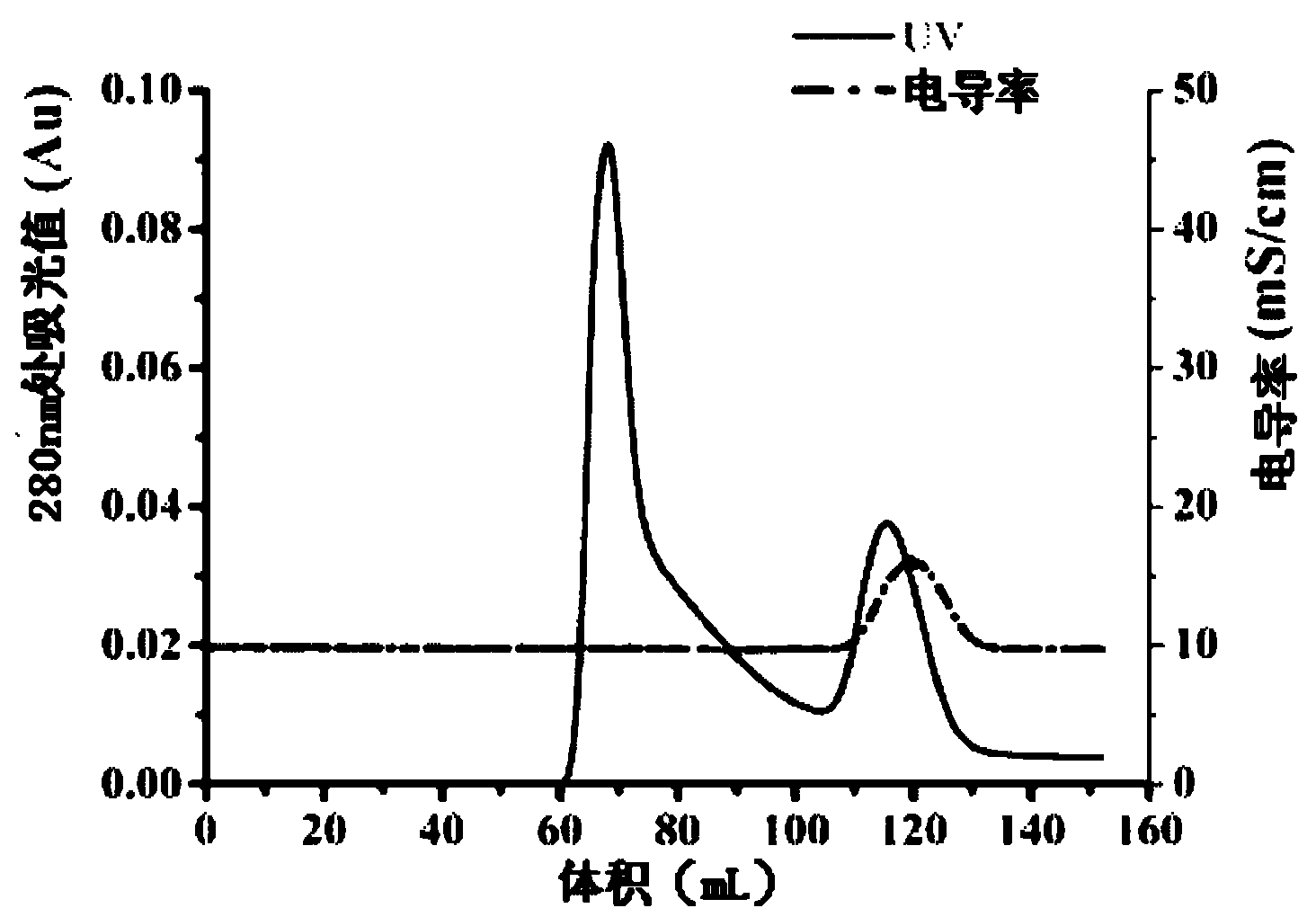
Expressing and purifying method of recombinant human-derived LECT2 protein in Pichia pastoris
A-LECT2, Pichia pastoris technology, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Expression and purification method of recombinant human LECT2 protein in Pichia pastoris of the present invention , The artificially synthesized human LECT2 gene was inserted into the extracellular expression vector pPICZαA; after enzyme digestion and linearization, electroporation was introduced into Pichia pastoris X33 to obtain recombinant engineering strains; after shake flask fermentation and methanol induction, the secretion of recombinant human LECT2 protein was realized Expression; and then purified by column chromatography to obtain a recombinant human LECT2 protein with a purity of more than 95%, the steps are as follows:
[0033] (1) Construction of recombinant expression plasmids
[0034]Extract RNA from human peripheral blood cells, reverse transcribe and synthesize cDNA to obtain the human LECT2 protein gene sequence, the accession number is NM002302, use this as a template to design primers for PCR amplification of human LECT2 protein, and the PCR amplifi...
Embodiment 2
[0044] The method for expressing and purifying the recombinant human LECT2 protein in Pichia pastoris of the present invention specifically comprises the following steps:
[0045] 1. Construction of human LECT2 gene Pichia pastoris expression vector
[0046] Total RNA was extracted from human peripheral blood cells, reverse-transcribed to synthesize cDNA, and primers were designed according to the published human source LECT2 gene sequence (NM_002302). The forward primer was 5'-GGAATTCGGGCCATGGGCTAATATATG-3', and the reverse primer was 5'-GGGTACCCAGGTATGCAGTAGGGTCAC-3'. The PCR reaction conditions were carried out according to the conventional settings. LECT2 was amplified by PCR, and the PCR product containing LECT2 was recovered with a gel-cutting recovery kit (OMEGA), and the recovered product was recovered with a restriction endonuclease Eco RI and Kpn I (Takara) double enzyme digestion, the digested PCR product was ligated with the same digested plasmid pPICZαA with T4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com