Method for recovering precious metal from waste aluminum-based catalyst and preparing high-purity alumina
A high-purity alumina and catalyst technology, applied in the direction of improving process efficiency, can solve problems such as long process flow, and achieve the effects of simple process operation, high added value of products, and easy scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
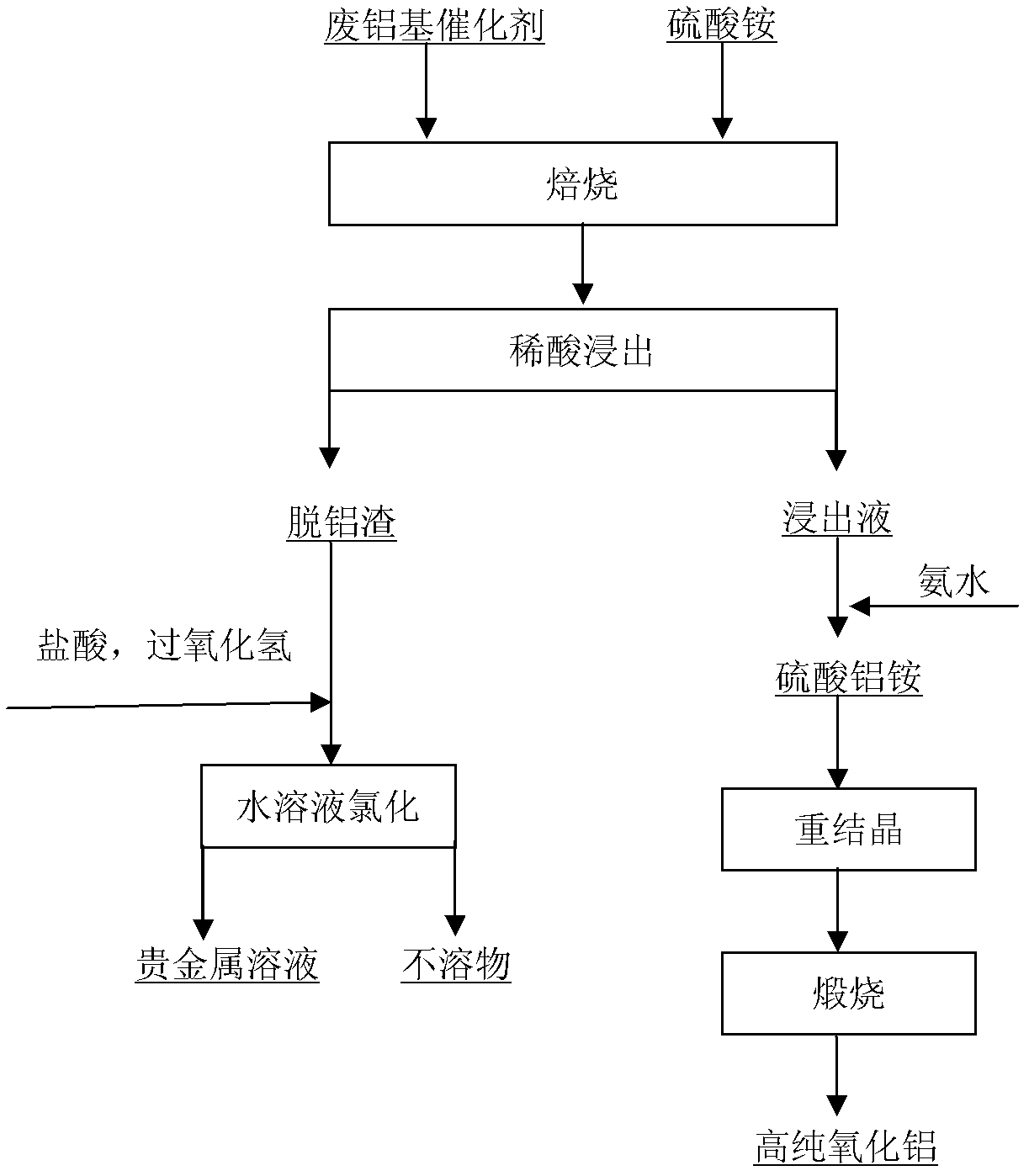
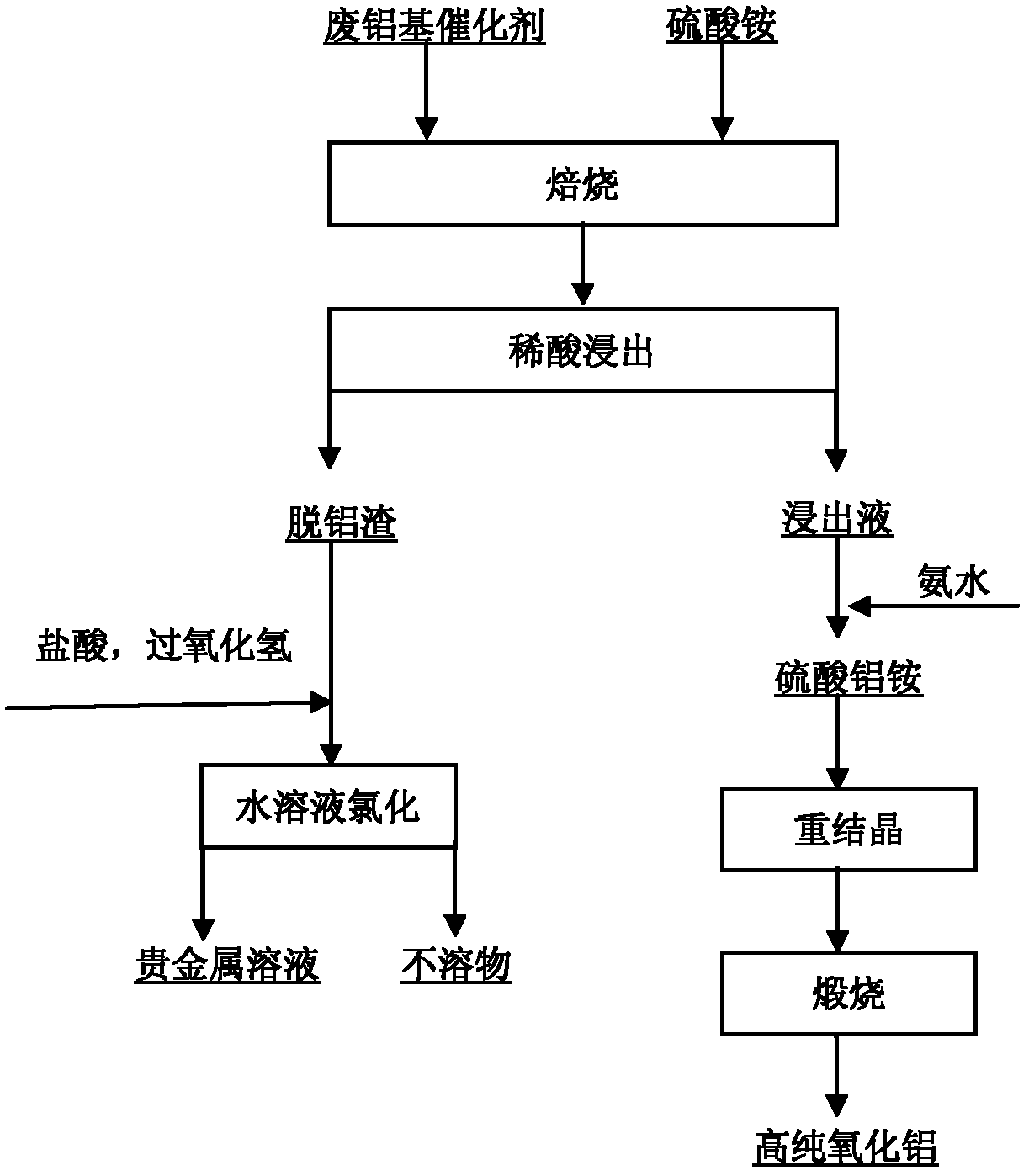
[0015] Embodiment 1, see attached figure 1 , take by weighing 100g waste aluminum-based catalyst (containing Pt 0.3%, finely ground) and 130g ammonium sulfate, fully mix, roast 2h at 300 ℃, roast product with dilute sulfuric acid (volume concentration 1mol / L) according to liquid-solid ratio 5: 1 (w / v) boiling leaching for 2h, after solid-liquid separation, the dealuminated slag adopts 6mol / L hydrochloric acid+hydrogen peroxide to dissolve 4h at 80 DEG C to reclaim platinum, and the Pt recovery rate is greater than 99%; Add ammoniacal liquor (mass Concentration 28%) to adjust the pH to 2, stir for a certain period of time, precipitate aluminum ammonium sulfate coarse crystals, repeat the recrystallization process 3 times, the obtained high-purity aluminum ammonium sulfate crystals were calcined at 1200 ° C for 5 hours, and high-purity alumina powder was obtained. Greater than 99.98%, the recovery rate is greater than 92%.
Embodiment 2
[0016] Embodiment 2, see attached figure 1 , Weigh 100g of waste aluminum-based catalyst (containing 0.3% of Pt) and 130g of ammonium sulfate, mix well, and roast at 400°C for 2h, and the roasted product is mixed with dilute sulfuric acid (1mol / L) at a liquid-solid ratio of 5:1 (w / v ) boiling leaching for 2 hours, after solid-liquid separation, the dealuminated slag was dissolved in 6mol / L hydrochloric acid+hydrogen peroxide at 80°C for 4 hours to recover platinum, and the recovery rate of Pt was greater than 99%; The pH is 2, stirred for a certain period of time, coarse crystals of ammonium aluminum sulfate are precipitated, and the recrystallization process is repeated 3 times. The obtained high-purity ammonium aluminum sulfate crystals are calcined at 1200°C for 5 hours to obtain high-purity alumina powder with a purity greater than 99.99%. The rate is greater than 92%.
Embodiment 3
[0017] Embodiment 3, see attached figure 1 , Weigh 100g of waste aluminum-based catalyst (containing 0.3% of Pt) and 150g of ammonium sulfate, mix well, and roast at 400°C for 4h, and the roasted product is mixed with dilute sulfuric acid (1mol / L) at a liquid-solid ratio of 5:1 (w / v ) boiling leaching for 2 hours, after solid-liquid separation, the dealuminated slag was dissolved in 6mol / L hydrochloric acid+hydrogen peroxide at 80°C for 4 hours to recover platinum, and the recovery rate of Pt was greater than 99%; The pH is 2, stirred for a certain period of time, coarse crystals of ammonium aluminum sulfate are precipitated, and the recrystallization process is repeated 3 times. The obtained high-purity ammonium aluminum sulfate crystals are calcined at 1200°C for 5 hours to obtain high-purity alumina powder with a purity greater than 99.99%. The rate is greater than 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com