Felodipine slow-release microspheres and preparation method thereof
A felodipine and gentle technology, applied in the direction of medical preparations with non-active ingredients, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of incomplete drug release and achieve increased blood drug concentration and oral biological Utilization, the effect of improving drug bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 O / W method prepares felodipine sustained-release microspheres
[0027] Table 1
[0028]
[0029] Preparation: Dissolve felodipine and EC in 100ml of dichloromethane according to the prescription in Table 1 as the organic phase (O). Then 5g of PVA emulsifier was dissolved in 500mL of water as the water phase (W). The organic phase is added into the water phase under high-speed stirring to homogeneously form an emulsion, and then the emulsion is poured into a large amount of water, the organic solvent is removed, the solid phase is separated by centrifugation, and the solid phase is dried to obtain the felodipine slow-release microspheres. The microsphere encapsulation efficiency is above 80%, and the particle size is 5-50 μm.
[0030]According to the quality standard of the United States Pharmacopoeia (USP35) felodipine sustained-release tablets, with PBS (pH6.5) containing 1% SDS as the release medium, the in vitro drug release assay was carried out, a...
Embodiment 2
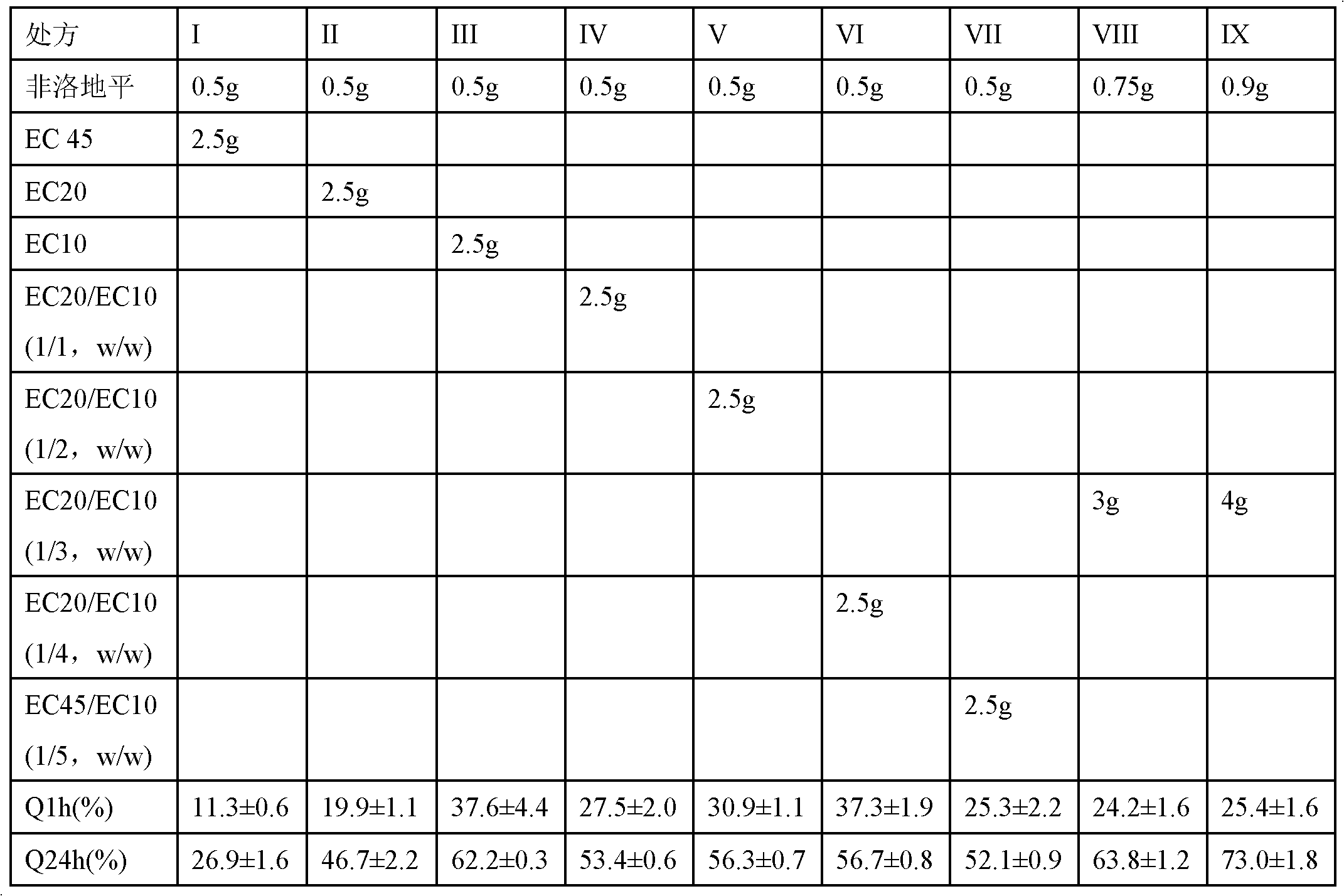
[0031] Embodiment 2 W / O / W method prepares felodipine sustained-release microspheres
[0032] Table 2
[0033] prescription
I
II
III
IV
V
VI
VII
0.5g
0.5g
0.5g
0.5g
0.5g
0.5g
0.5g
EC45
2.5g
2.5g
2.5g
2.5g
2.5g
2.5g
2.5g
water
0.1g
0.1g
0.1g
0.1g
0.1g
PEG400
0.1g
Q1h(%)
25.3±0.7
24.4±1.3
34.8±6.4
25.4±1.3
27.9±1.5
39.9±2.6
23.3±2.2
Q24h(%)
89.3±2.7
86.1±0..7
86.3±5.6
83.3±5.0
82.8±1.6
89.4±2.0
93.4±0.7
[0034] Preparation: Dissolve sucrose, mannitol, lactose, glycine, sodium...
Embodiment 3
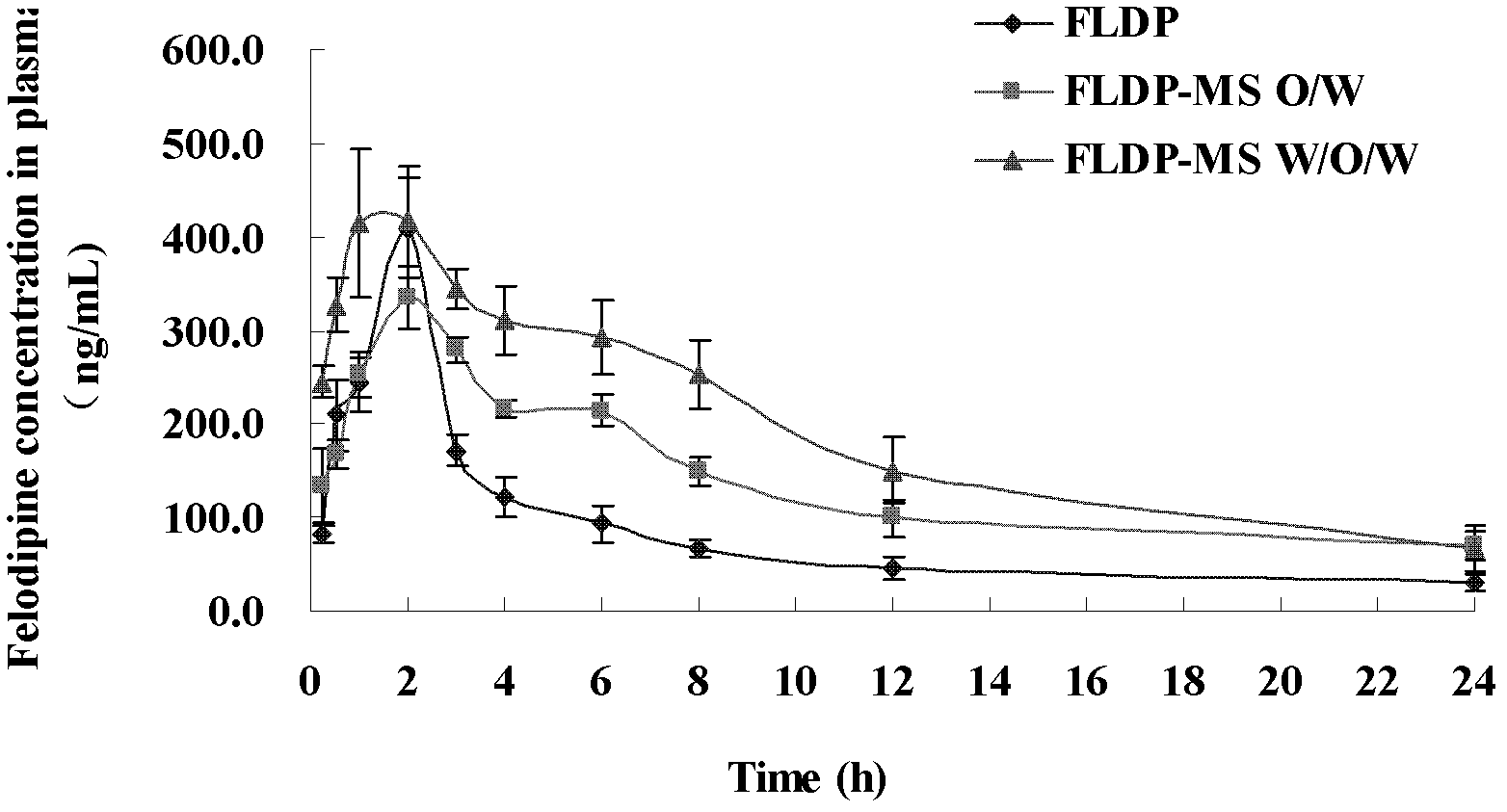
[0036] Embodiment 3: The pharmacokinetic comparison of felodipine microspheres prepared by W / O / W method and O / W method and felodipine suspension
[0037] 180 ICR mice, half male and half female, weighing 18-20 g, were randomly divided into 30 groups, 6 mice in each group. Felodipine microspheres (prescription IX in embodiment 1) prepared by O / W method, felodipine microspheres (prescription VII in embodiment 2) prepared by W / O / W method, and felodipine bulk drug are mixed with A suspension containing felodipine at a concentration of 1 mg / ml. Mouse fasting 12h, the felodipine microsphere (prescription IX in the embodiment 1) that the O / W method of 150mg / kg of felodipine dosage is prepared by intragastric administration respectively, the felodipine microsphere prepared by W / O / W method Ball (prescription VII in embodiment 2), and felodipine suspension, after administration 15min, 30min, 1h, 2h, 3h, 4h, 5h, 6h, 8h, 12h and 24h, orbital blood is taken in sodium heparin In the treat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com