Doxycycline hydrochloride dual-release preparation and preparation method thereof
A doxycycline hydrochloride, double-release technology, applied in the direction of tetracycline active ingredients, pharmaceutical formulations, drug delivery, etc., to achieve the effects of reducing side effects and addiction, improving stability, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Doxycycline hydrochloride double-release capsules and preparation method thereof
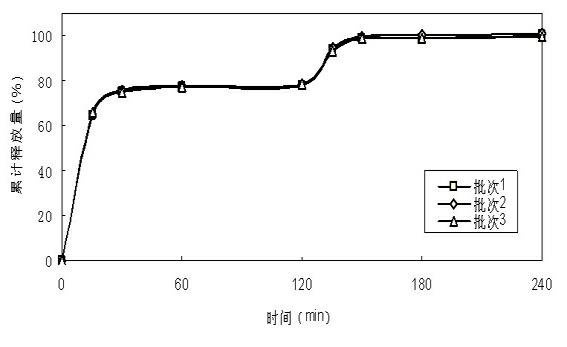
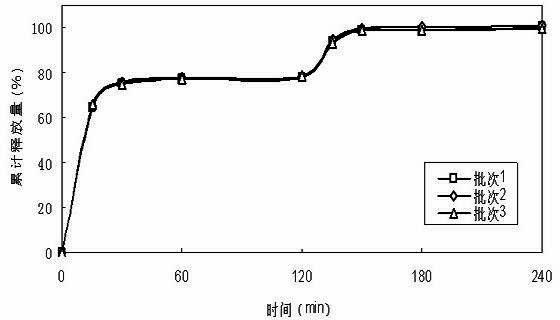
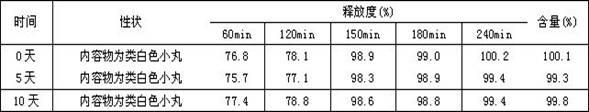
[0044] First prepare the drug-containing pellet cores according to the formula, prepare immediate-release pellets from the pellet cores, and then prepare delayed-release pellets from the immediate-release pellets, and finally fill the capsules with the immediate-release pellets and the delayed-release pellets in proportion, namely Obtain doxycycline hydrochloride double-release capsules.
[0045] (1) Ball core preparation process
[0046] Ball core prescription: microcrystalline cellulose 640g, doxycycline hydrochloride 177g, 2% (w / w) hydroxypropyl methylcellulose solution 640g.
[0047] Preparation process: pulverize doxycycline hydrochloride and filler microcrystalline cellulose to a certain particle size range, pass through an 80-mesh sieve, mix in a stirring mixer for 30 minutes, add 2% hydroxypropyl methylcellulose solution, Made of soft materials. Turn on the low-temp...
Embodiment 2
[0062] Example 2 Doxycycline hydrochloride double-release capsules and preparation method thereof
[0063] (1) Ball core composition: 640g of microcrystalline cellulose, 177g of doxycycline hydrochloride, 640g of 2% (w / w) povidone solution.
[0064] Preparation process: pulverize doxycycline hydrochloride and microcrystalline cellulose, pass through an 80-mesh sieve, mix in a stirring mixer for 30 minutes, then add povidone solution to make a soft material. Turn on the low-temperature refrigerator and control the temperature at 4-15°C. After 10 minutes, put the soft material into the low-temperature and high-pressure extruder to pass through the sieve plate with a sieve hole of 0.9-1.0mm, and adjust the extrusion speed to 40rpm to form a cylindrical shape with a moderate length. Soft materials are spheronized in a spheronizer at a speed of 1800rpm for 10 minutes to form drug-loaded pellets with uniform particle size. According to the length of the sheared cylinder, the parti...
Embodiment 3
[0072] Example 3 Doxycycline hydrochloride double-release capsules and preparation method thereof
[0073] (1) Pill core composition: 640 g of microcrystalline cellulose, 177 g of doxycycline hydrochloride, 20 g of crospovidone, and 640 g of 2% hydroxypropyl methylcellulose aqueous solution.
[0074] Preparation process: pulverize doxycycline hydrochloride, microcrystalline cellulose and crospovidone, pass through an 80-mesh sieve, mix in a stirring mixer for 30 minutes, add 640g of hydroxypropylmethylcellulose aqueous solution, and make soft material. Turn on the low-temperature refrigerator and control the temperature at 4-15°C. After 10 minutes, put the soft material into the low-temperature and high-pressure extruder and pass through the sieve plate with a size of 0.9-1.0mm. Adjust the extrusion speed to 40rpm to form a medium-length cylindrical shape. The soft material is spheronized in a spheronizer at a speed of 1800rpm for 10 minutes to form drug-loaded pellets with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com