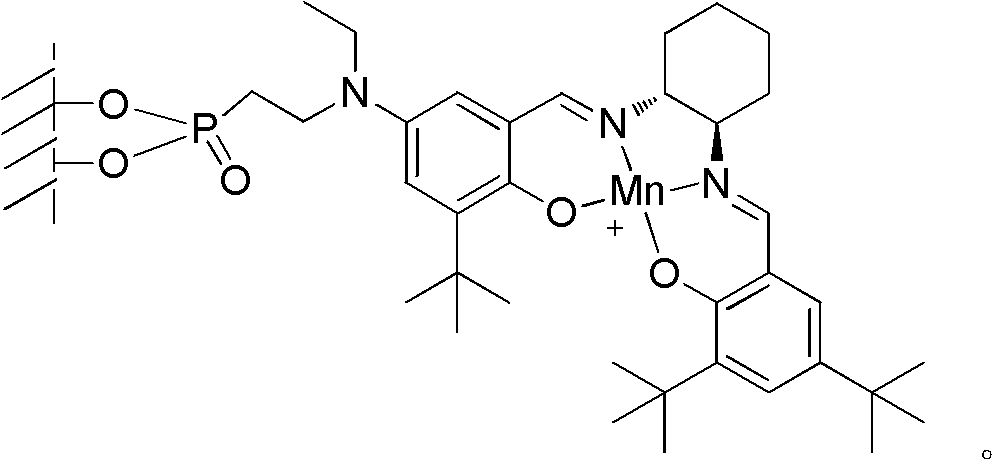
Loaded type salen manganese complex catalyst, preparation method and application thereof
A manganese complex, supported technology is applied to the supported salen manganese complex catalyst and the field of preparation and application thereof, which can solve the problems of high cost, low catalytic activity, only 20%, etc., and achieves convenient recovery, high catalytic activity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, a kind of preparation method of loaded type salen manganese complex catalyst, carries out following steps successively:
[0043] 1), γ-alumina surface treatment:
[0044] A. Add 10g of γ-alumina and 0.2g of sodium to 50mL of toluene, stir and reflux for 1.5 hours;
[0045] B. Cool the mixed solution obtained in step A to 5°C, add 1g of 2-chloroethylphosphonyl chloride in 10mL toluene solution dropwise, drop it in about 15 minutes, continue to stir at 5°C for 2 hours after the drop, filter, and the obtained filter Cake I was washed with clear water and then dried (50°C, dried to constant weight), to obtain filter cake I after drying;
[0046] C, filter cake I and 30g ethylamine are reacted 4 hours under the MPa pressure of 0.1~0.5 in autoclave after drying, reaction temperature 50~55 ℃;
[0047] After the reaction is completed, cool down to room temperature, filter, wash the filter cake II with water and then dry (50°C, dry to constant weight); obtain the...
Embodiment 2
[0055] The epoxidation of embodiment 2, styrene
[0056] Add 10 mL of water, 1 mmol of styrene, 0.08 mmol of pyridine N-oxide and 50 mg of the supported salen manganese complex catalyst prepared in Example 1 (that is, about 0.02 mmol of the Salen manganese complex) into the reaction kettle, and slowly drop Add 1.5 mmol of hydrogen peroxide with a mass concentration of 15%, drop it in about 1 hour, and continue the reaction at 0° C. for 5 hours after the drop.
[0057] The obtained reaction product was separated and processed by column chromatography to obtain 120 mg of styrene oxide with a purity of 99%.
[0058] The yield of styrene oxide was 99%, and the ee value was 72%.
Embodiment 3
[0059] The epoxidation of embodiment 3, cyclohexene
[0060] Add 10mL of water, 1mmol of cyclohexene, 0.08mmol of pyridine N-oxide and 50mg of the loaded salen manganese complex catalyst prepared in Example 1 in the reaction kettle, and slowly add 1.5mmol of a 25% mass concentration at 0°C Hydrogen peroxide, drop in about 1 hour, and continue to react at 0°C for 6 hours after the drop.
[0061] The obtained reaction product was separated and processed by column chromatography to obtain 95 mg of epoxycyclohexane with a purity of 99%.
[0062] The yield of epoxycyclohexane was 96%, and the ee value was 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com