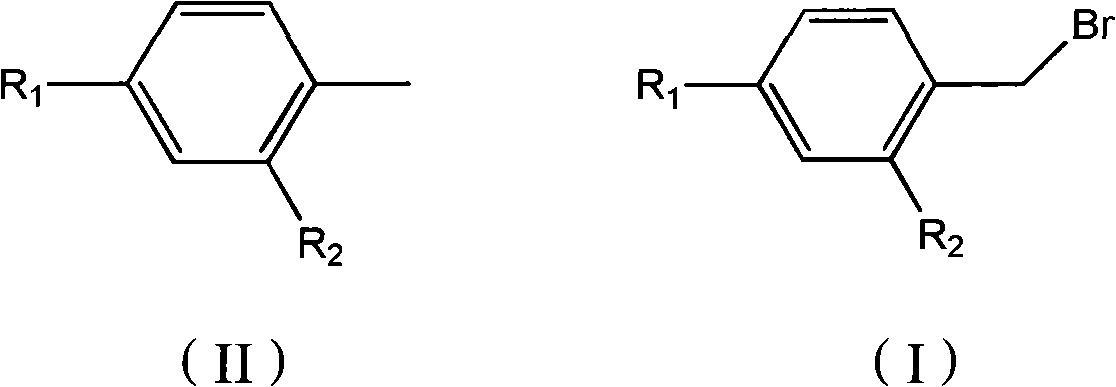
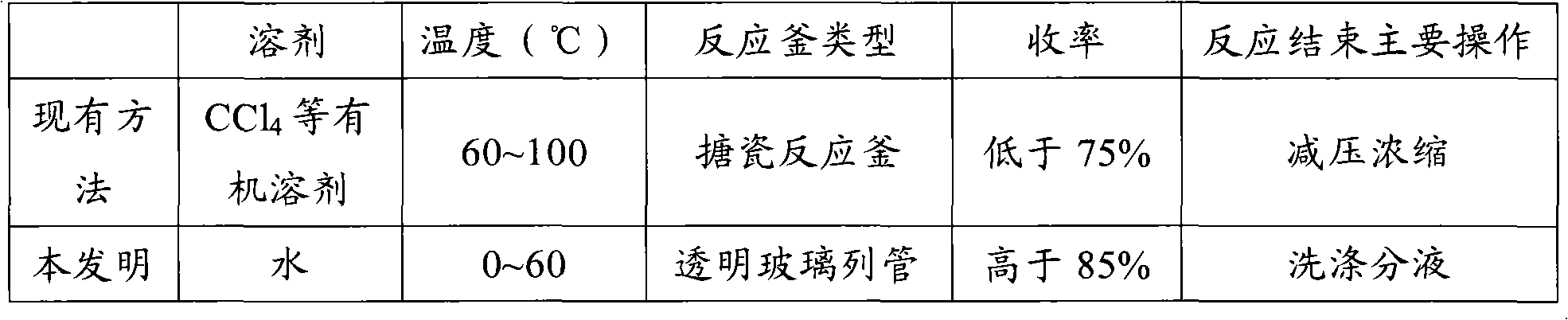
Preparation method for bromine benzyls compound
A compound, bromosuccinimide technology, applied in the field of organic synthesis, can solve the problems of long time period, complicated operation, low selectivity, etc., and achieve short reaction time period, mild reaction conditions and good reaction selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the preparation of 4-acetyl benzyl bromide
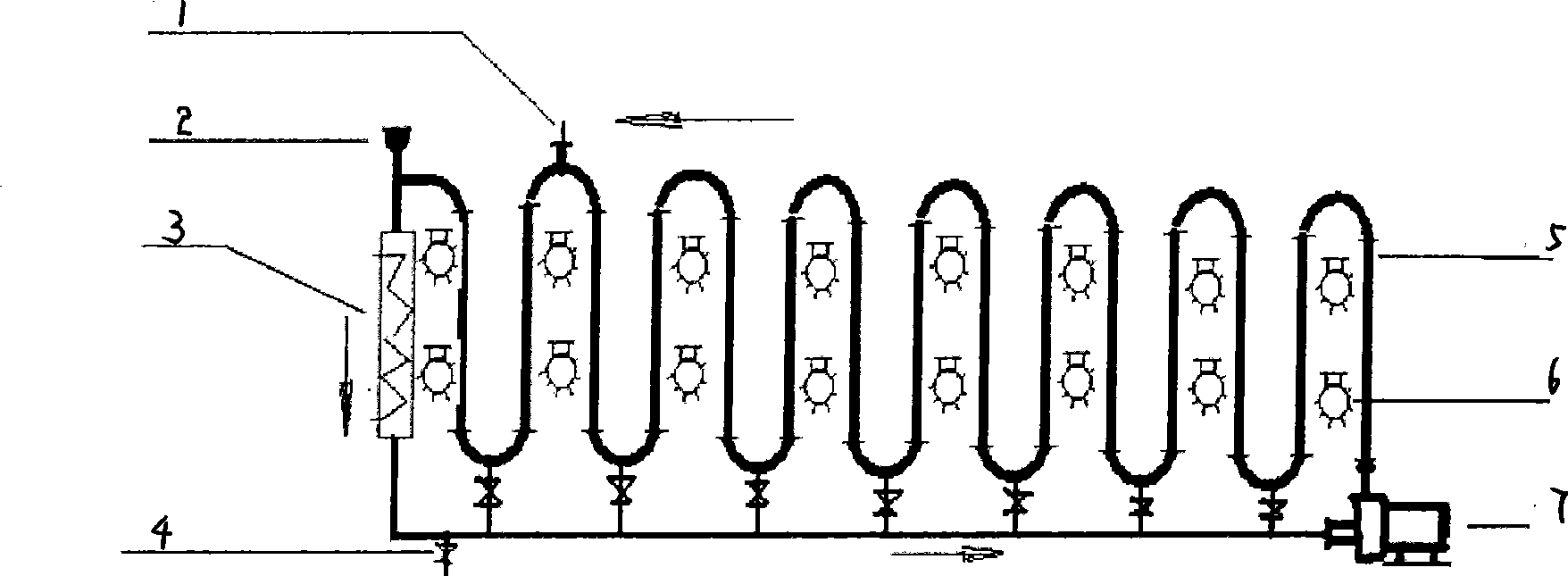
[0017] exist figure 1 In the photoreaction equipment shown, add 10kg p-methylacetophenone, 40kg water by feeding system 2, open circulation pump 7 and make reaction system form turbulent flow in fully transparent tube 5, turn on 10 lamps of 105w (that is, High-power lighting 6), the temperature is maintained at 20-50°C by the temperature control system 3, and then 13.6kg NBS and 0.5kg AIBN are added by the feeding system 2, and the reaction is terminated after 12 hours. Emit whole reaction liquid from ball valve 4, use the NaHCO that mass fraction is 10% 3 The reaction liquid was washed with aqueous solution, and the lower organic phase was collected by liquid separation, then heated and dissolved with ethanol until clarified, and 14.5 kg of light yellow crystals were precipitated by cooling down, with a yield of 91.7% and a purity of 97% by HPLC. The melting point is 36-38°C. 1 H NMR (600MHz, CDCl 3 )δ: 7...
Embodiment 2
[0018] Embodiment 2: the preparation of 4-tert-butylbenzyl bromide
[0019] exist figure 1 In the photoreaction equipment shown, add 15kg 4-tert-butyltoluene, 35kg water by feeding system 2, open circulation pump 7 and make reaction system form turbulent flow in fully transparent tube 5, turn on 10 lamps of 105w (that is, High-power lighting 6), the temperature is maintained at 20-50°C by the temperature control system 3, and then 18kg NBS is added from the feeding system 2, and the reaction is terminated after 4 hours without a catalyst. Emit whole reaction liquid from ball valve 4, use the NaHCO that mass fraction is 10% 3 The reaction solution was washed with aqueous solution, the lower organic phase was collected by liquid separation, and then washed with n-hexane to obtain 20.5 kg of colorless oily liquid with a yield of 89.1% and a purity of 96% by HPLC. no D 20 = 1.5448; 1 H NMR (600MHz, CDCl 3 )δ: 7.56 (d, J = 7.8Hz, 2H), 7.43 (d, J = 7.8Hz, 2H), 4.46 (s, 2H), ...
Embodiment 3
[0020] Embodiment 3: the preparation of 4-methoxybenzyl bromide
[0021] exist figure 1 In the photoreaction equipment shown, add 20kg 4-methoxytoluene, 40kg water by feeding system 2, open circulation pump 7 and make reaction system form turbulent flow in fully transparent tube 5, turn on 10 lamps of 105w (that is, High-power lighting 6), the temperature is maintained at 20-50°C by the temperature control system 3, and then 13.6kg NBS and 0.5kg AIBN are added from the feeding system 2, and the reaction is completed after 15 hours of reaction. Emit whole reaction liquid from ball valve 4, use the NaHCO that mass fraction is 10% 3 The reaction liquid was washed with aqueous solution, the lower organic phase was collected by liquid separation, and then washed with n-hexane to obtain 20.5 kg of oily liquid with a yield of 85.5% and a purity of 95% by HPLC. no D 20 = 1.5819; 1 H NMR (600MHz, CDCl 3 )δ: 7.43 (d, J = 7.8 Hz, 2H), 7.12 (d, J = 7.8 Hz, 2H), 4.46 (s, 2H), 3.83 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com