Production method for medical grade valine
A production method and technology for valine, which are applied in the field of separation and purification of amino acids, can solve the problems of complex process, reduced yield, loss of regeneration ability and the like, and achieve the effects of simple equipment and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
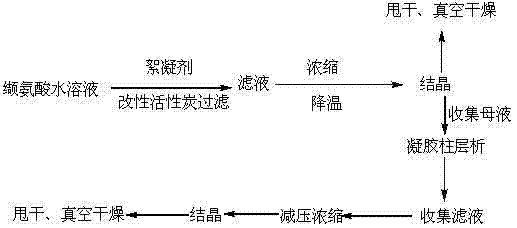
[0027] Preparation of modified activated carbon: Soak activated carbon in PH=6, 30% aqueous hydrogen peroxide solution by weight for 10 hours at room temperature, filter, wash with pure water until the washing liquid has no acid ions, dry, and then dry at 200°C Dry and activate for 6 hours to obtain modified activated carbon.
[0028] Pump 1000L of pure water into the dissolution tank, heat it to 60°C, add 100.40Kg of industrial valine raw material, start stirring until it is completely dissolved, keep it warm for about 6 hours, add 10.02Kg of polyacrylamide flocculant (Shanghai Minaqing Industry and Trade Co., Ltd. Produced by the company, the model is Kingfloc4170 polyacrylamide), stirred for 3 hours, filtered to remove the flocculant and its adsorbed proteins, microorganisms and debris, filtered, added 14.50Kg modified activated carbon to the filtrate, stirred for 3 hours to decolorize and remove bacterial endotoxin , Filtration, the filtrate is hot-filtered with an ultrafi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com