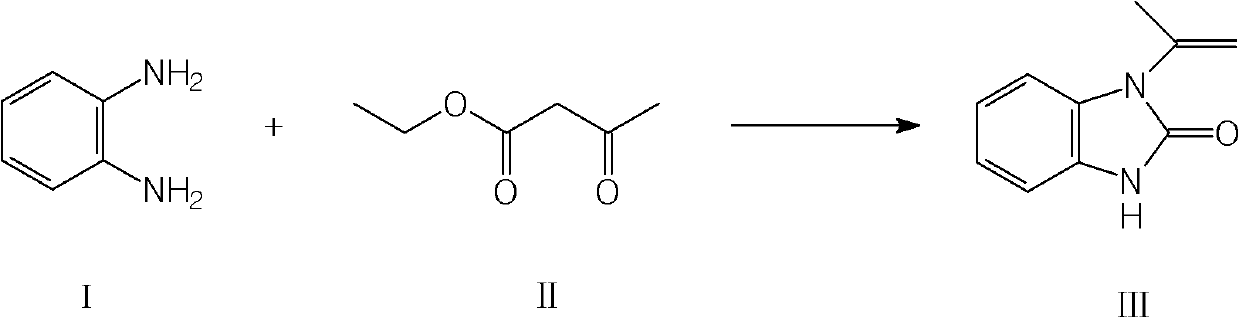
Preparation method of 1-isopropenyl-2-benzimidazolone
A benzimidazolone and isopropenyl technology, which is applied in the field of preparation of main raw material 1-isopropenyl-2-benzimidazolone, can solve the problems of high cost, difficult industrialized production, difficult control and the like, and achieves the production cost The effect of reducing and increasing costs and eliminating potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 1000ml four-necked flask equipped with a distiller, add 480g xylene, 108g o-phenylenediamine, 1.5g methylguanidine, then add 150g ethyl acetoacetate, raise the temperature to 125-135°C, and react at this temperature During 7 hours, part of the mixture of by-product alcohol and water was evaporated, xylene was distilled under reduced pressure, and 650ml of 2% hydrochloric acid was added to the obtained crude product, and it was beaten for 1 to 2 hours at 25°C at room temperature, suction filtered, washed with 400g of water, 60 ~80°C, 150~250mbar drying to constant weight to obtain 148g of the target product 1-isopropenyl-2-benzimidazolone.
[0038] It was tested to be consistent with the standard of 1-isopropenyl-2-benzimidazolone.
[0039] HPLC: 98.76%
[0040] mp: 125-125.5°C, C, 62.5; H, 6.2; N, 14.6, yield: 85%.
Embodiment 2
[0042] In a 1000ml four-neck flask equipped with a distiller, add 450g xylene, 100g o-phenylenediamine, 1.4g methylguanidine, then add 160g ethyl acetoacetate, raise the temperature to 125-135°C, and react at this temperature During 8 hours, part of the mixture of by-product alcohol and water was distilled off, xylene was distilled under reduced pressure, and 600ml of 10% hydrochloric acid was added to the obtained crude product, beaten at 30°C for 1 hour, suction filtered, washed with 600g of water, 60-80°C, After drying at 150-250 mbar, 134.5 g of the target product 1-isopropenyl-2-benzimidazolone was obtained.
[0043] Purity HPLC: 97.23%
[0044] mp: 123.5-125.5°C, C, 62.4; H, 6.3; N, 14.6, yield: 83.5%.
Embodiment 3
[0046] In a 1000ml four-necked flask equipped with a distiller, add 420g xylene, 110g o-phenylenediamine, 1.8g triethylamine, then add 135g ethyl acetoacetate, raise the temperature to 125-135°C, and react at this temperature During 9 hours, part of the mixture of by-product alcohol and water was distilled off, xylene was distilled under reduced pressure, and the obtained crude product was added with 600ml of 5% hydrochloric acid, beaten at room temperature for 1 hour, suction filtered, washed with 600g of water, 60~80℃, 150~ Dry at 250 mbar to constant weight to obtain 142 g of the target product 1-isopropenyl-2-benzimidazolone, mp: 124-125.5°C, C, 62.5; H, 6.3; N, 14.5, yield: 81.5%. Purity HPLC: 97.86%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com