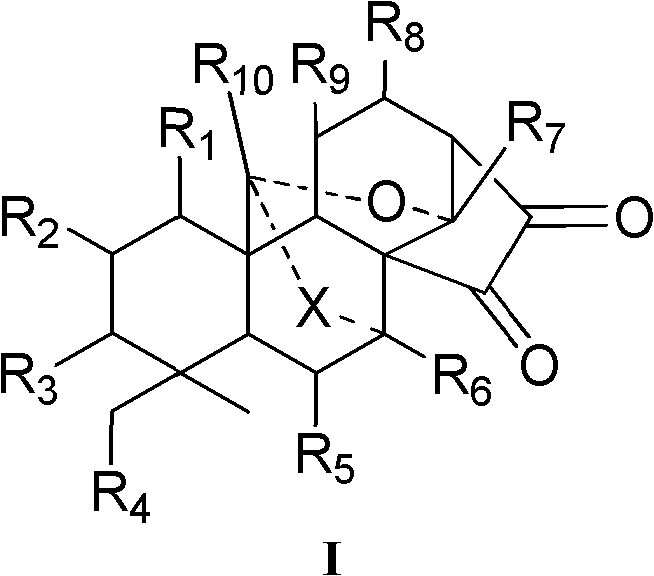
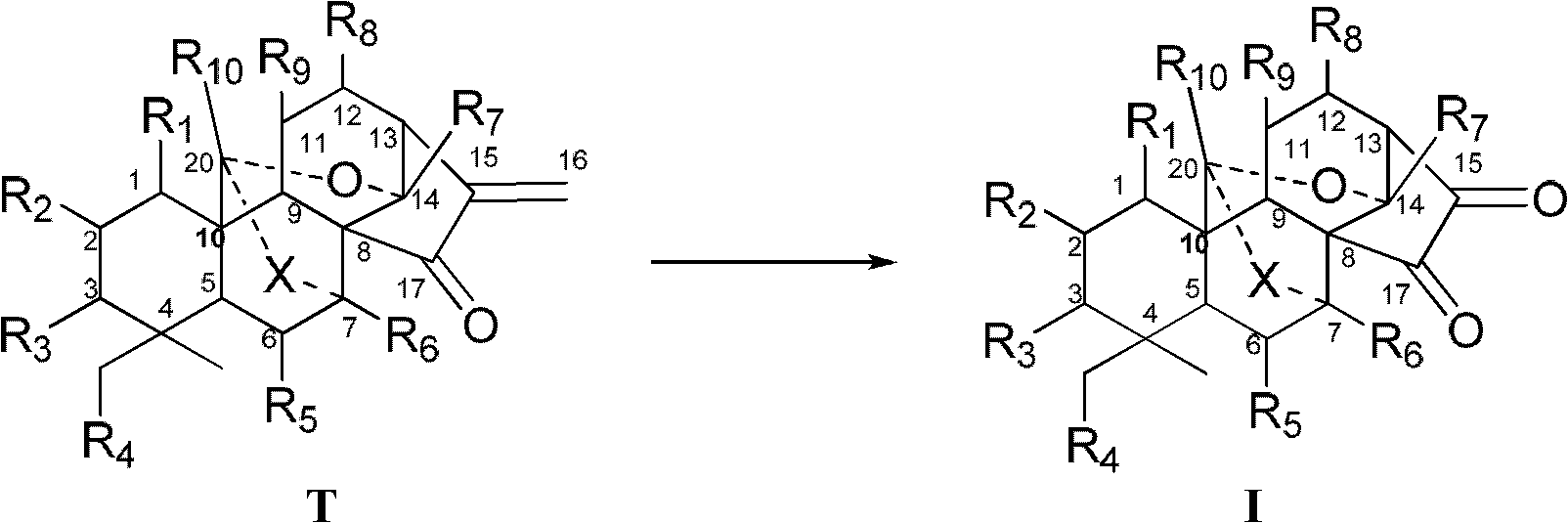
Enantiomorphous-kaurene diterpene and derivative and preparation method thereof
A technology of kaurane type and diterpene, which is applied in the field of preparation of en-kaurene type diterpene and its derivatives, and can solve the problems of insufficient chemical methods and low synthesis efficiency of derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
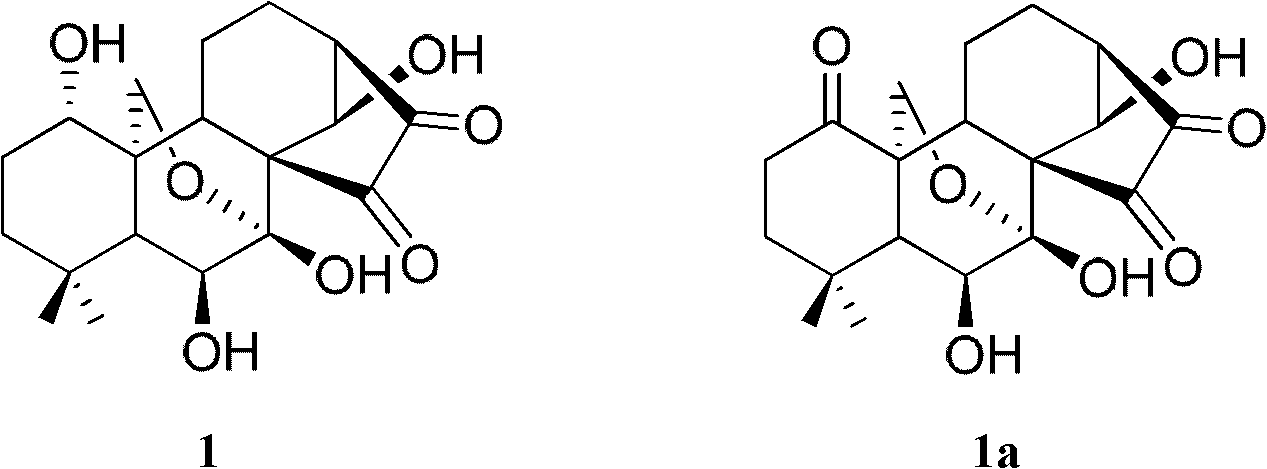
[0125] Example 1 Synthesis of Oridonin A Cyclobutanedione
[0126] (1) Synthesis of oridonin cyclobutanedione (i.e. compound 1)
[0127]
[0128] Get Rubescensine A (1.5g, 4.12mmol; Specification: 95%, purchased from Xi'an Haoxuan Biotechnology Co., Ltd.) and dissolve it in dichloromethane (100mL) and methanol (20mL), cool in an ice-salt bath and control the internal temperature. Temperature (-5-15°C), blow oxygen containing ozone under the liquid surface of the above system at a speed of 2-4L / min (BGF-YQ type ozone generator, 2g / h, 60W, Beijing Beili Guoke Ozone Application Technology Co., Ltd.), and continue blowing for 0.5-1.5h. At this time, the color of the system is a yellow solution, and TLC detects that the raw materials have reacted completely (the developer is: chloroform / acetone / methanol=8 / 1 / 1(v / v / v), 10 wt% phosphomolybdic acid color). Then, excess ozone in the reaction system was removed by bubbling nitrogen gas, and dimethyl sulfide (1.5 mL) was added to the ...
Embodiment 2
[0136] Example 2 Synthesis of Oridonin Pyrazine Derivatives
[0137]
[0138] Compound 1 (200mg, 0.55mmol) was dissolved in methanol (15mL), o-phenylenediamine (300mg, 2.78mmol) was added to the resulting solution at room temperature, and reacted overnight with stirring, the system became a brownish yellow solution, and the reaction of raw materials was detected by TLC. completely. After the reaction system was concentrated, the residue obtained was purified by silica gel column chromatography (chloroform / methanol=25 / 1, 20 / 1, by volume ratio), and the eluent from the product point was collected and concentrated to obtain compound 2 as a light yellow solid. (180 mg), molar yield: 75%.
[0139] 1 H NMR (300MHz, DMSO-d 6 ): δ1.17(s, 3H), 1.20(s, 3H), 1.92(m, 1H), 2.43(m, 2H), 3.26(m, 2H), 3.67(dd, J=6.0, 9.9Hz, 1H), 3.97, 4.27(2d, J=9.9Hz, each 1H), 4.34(d, J=4.8Hz, 1H), 5.17(s, 1H), 6.22(s, 1H), 6.84(s, 1H) , 7.71 (d, J = 10.2 Hz, 1H), 7.79 (m, 2H), 7.92 (m, 1H), 8.03 (m...
Embodiment 3
[0178] Example 3 Synthesis of Rubescensine A Dihydropyrazine Derivatives
[0179]
[0180] Using ethylenediamine as a raw material, referring to the operation of Example 2 (1), Compound 15 was obtained.
[0181] 1 H NMR (300MHz, CDCl 3 ): δ1.03-1.08(2s, 6H), 1.88(m, 1H), 2.05, 2.44(2m, 2H), 2.87(d, J=10.5Hz, 1H), 3.43-3.67(m, 6H), 3.71 (d, J=6.6Hz, 1H), 4.09, 4.29 (2d, J=10.2Hz, each 1H), 4.89 (s, 1H), 5.05, 6.00, 7.98 (3br s, 3H). ESI-MS (m / z): 391.2 (M+H)+ (C 21 h 31 N 2 o 5 , theoretical value: 391.22).
[0182]
[0183] Using o-diaminocyclohexane as a raw material, refer to the operation of Example 2 (1) to obtain compound 16.
[0184] 1 H NMR (300MHz, DMSO-d 6 ): δ1.02-1.08(2s, 6H), 2.06(m, 1H), 2.58-2.61(m, 2H), 3.17-3.25(m, 2H), 3.40-3.48(m, 3H), 3.86, 4.11 (2d, J=9.9Hz, each 1H), 4.31-4.33 (m, 1H), 4.73 (s, 1H), 5.86-6.03 (m, 1H), 6.63-7.51 (m, 2H). ESI-MS (m / z): 445.2 (M+H) + (C 25 h 37 N 2 o 5 , theoretical value: 445.27).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com