Polyurethane segmented copolymer containing disulfide bonds and tertiary amine groups as well as preparation method of polyurethane segmented copolymer
A technology of block copolymer and polyurethane, applied in the field of polyurethane, can solve problems such as unreported, and achieve the effect of simple and easy method, easy control and good medical application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
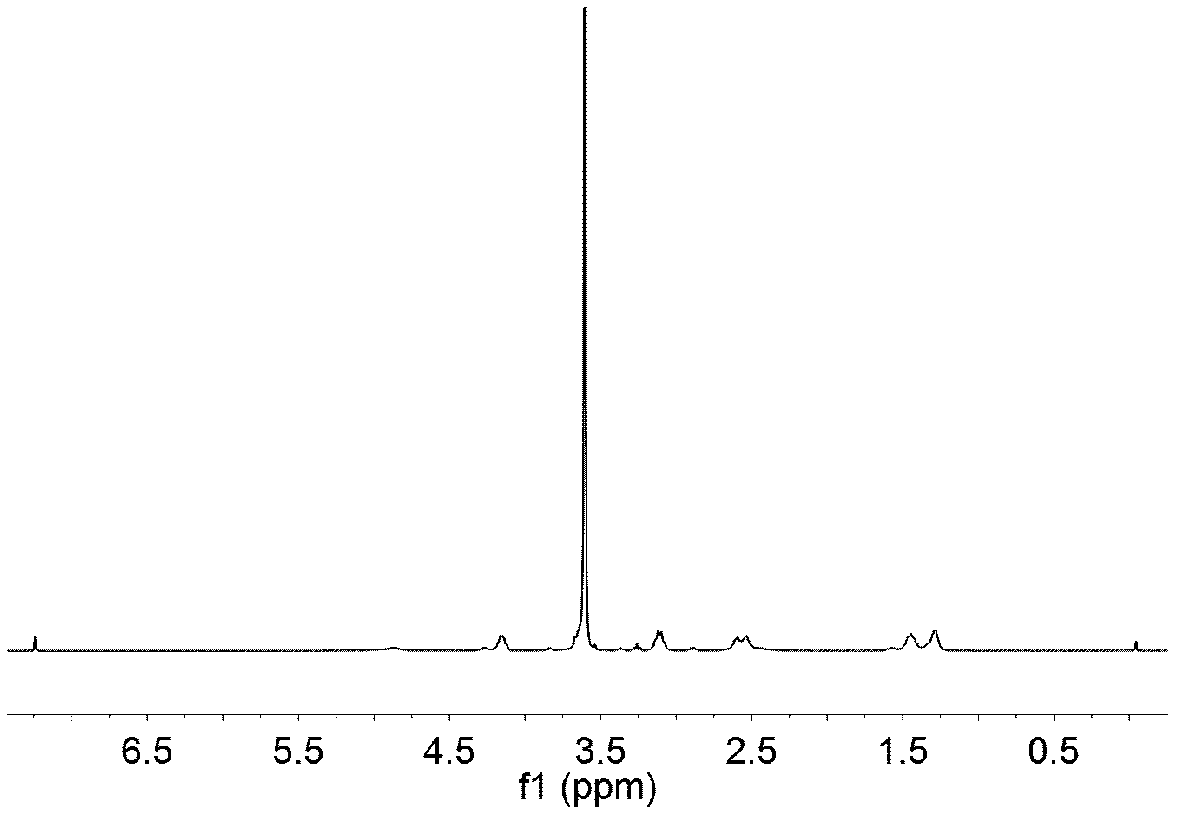
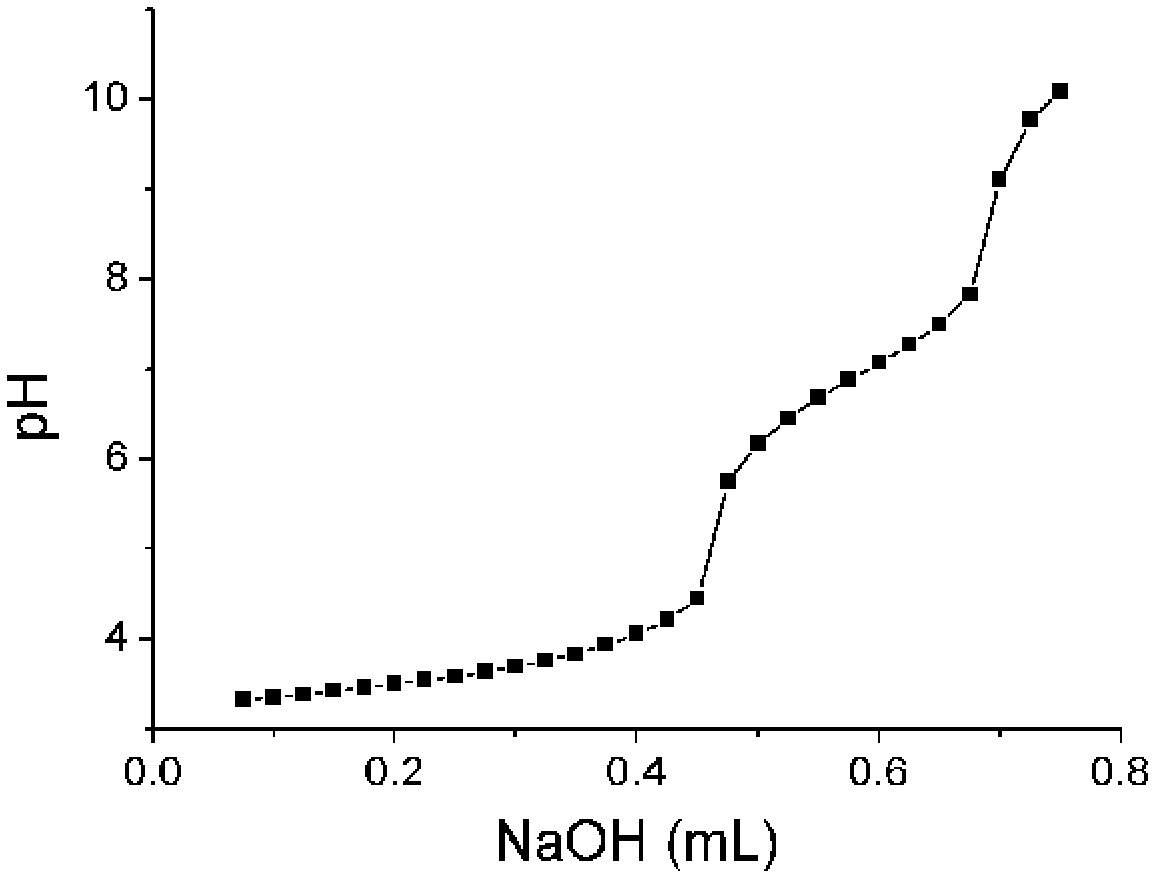
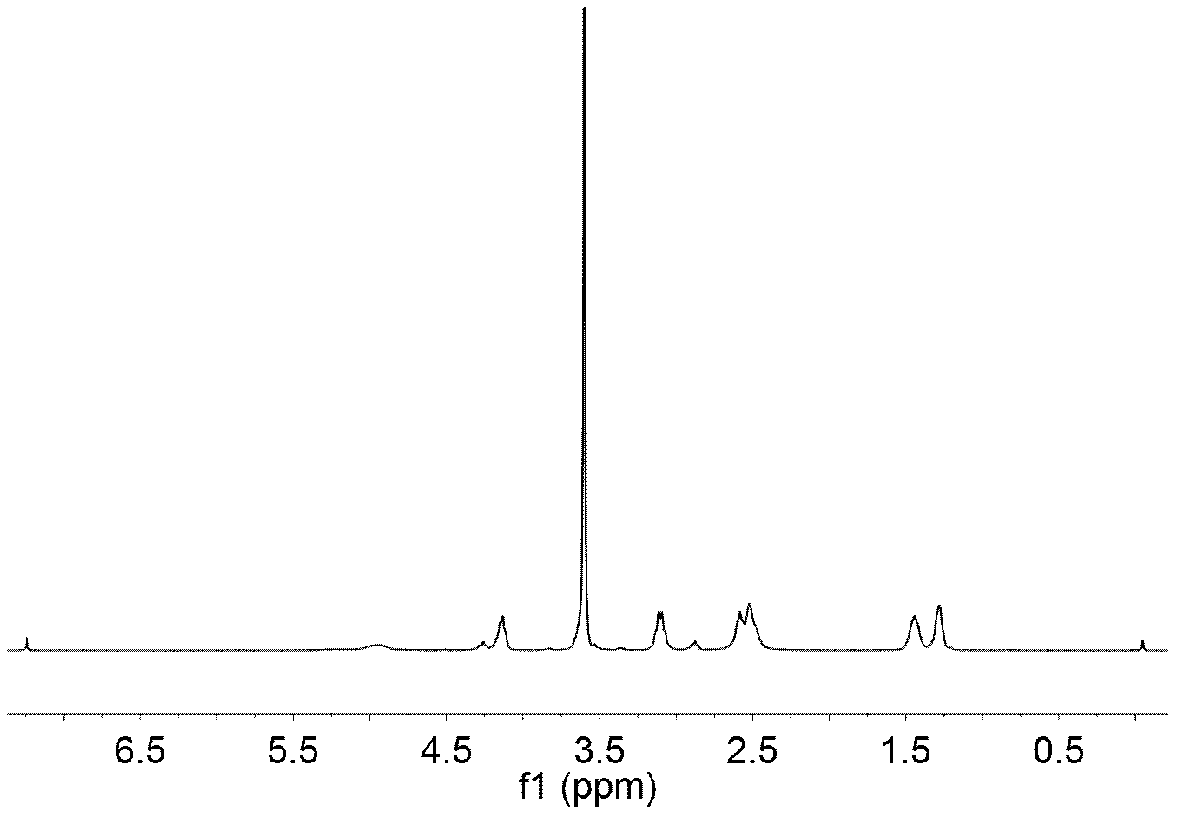
Image
Examples
preparation example Construction
[0070] Correspondingly, the present invention provides a kind of preparation method of the polyurethane block copolymer described in above-mentioned technical scheme, comprises the following steps:
[0071] Under the action of a catalyst, react diols containing disulfide bonds, diols containing tertiary amines, and diisocyanates in 1,2-dichloroethane for 10 to 60 minutes, and then add polyethylene glycol monomethyl ether to continue the reaction 2~10h, obtain ABA type polyurethane block copolymer,
[0072] The number average molecular weight of the polyethylene glycol monomethyl ether is 500-10000, preferably 1500-8000, more preferably 2000-5000; the diol containing disulfide bonds, diol containing tertiary amine and diol The ratio of the total mass of isocyanate to the mass of polyethylene glycol monomethyl ether is (1~9): (1~9), preferably (3~7): (3~7), more preferably (4~6 ): (4~6); The mass ratio of the diol containing disulfide bond and the diol containing tertiary amine...
Embodiment 1~12
[0088] Add a certain amount of PEG into a dry reaction flask, add anhydrous toluene, and azeotropically remove water at 130°C for 6 hours, then drain the remaining toluene under reduced pressure, and cool to room temperature under nitrogen protection;
[0089] Then add DHDS, HEP, the catalyst dibutyltin dilaurate and an appropriate amount of 1,2-dichloroethane into the reaction flask containing PEG, and stir at 75°C until the solid dissolves;
[0090] Then HDI was added into the reaction flask, and stirred and reacted for 5 hours at 75°C under nitrogen protection;
[0091] The reaction product was settled with ether, and then the obtained solid was dissolved in chloroform, then settled with ether, filtered with suction, and dried to obtain the product.
[0092] The formula of specific raw material monomers is shown in Table 1, and the molecular weight characterization of the polyurethane obtained in Examples 2 to 6 is shown in Table 2.
[0093] To measure the performance of t...
Embodiment 13~21
[0095] Add a certain amount of PEG with a number average molecular weight of 2000 into a dry reaction flask, add anhydrous toluene, and azeotropically remove water at 130°C for 8 hours, then drain the remaining toluene under reduced pressure, and cool under nitrogen protection conditions to room temperature;
[0096] Then add DHDS, TCI-EP, catalyst stannous octoate and appropriate amount of 1,2-dichloroethane into the reaction flask containing PEG, and stir at 75°C until the solid dissolves;
[0097] Then HDI was added into the reaction flask, and stirred and reacted for 5 hours at 75°C under nitrogen protection;
[0098] The reaction product was settled with ether, and then the obtained solid was dissolved in chloroform, then settled with ether, filtered with suction, and dried to obtain the product. (the formula of concrete raw material monomer sees Table 1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com