Catalyst for carbon dioxide methanation and preparation method thereof
A technology of carbon dioxide and catalyst, which is applied in the field of carbon dioxide methanation catalyst and its preparation, achieving the effects of low energy consumption, small particle size and uniform dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
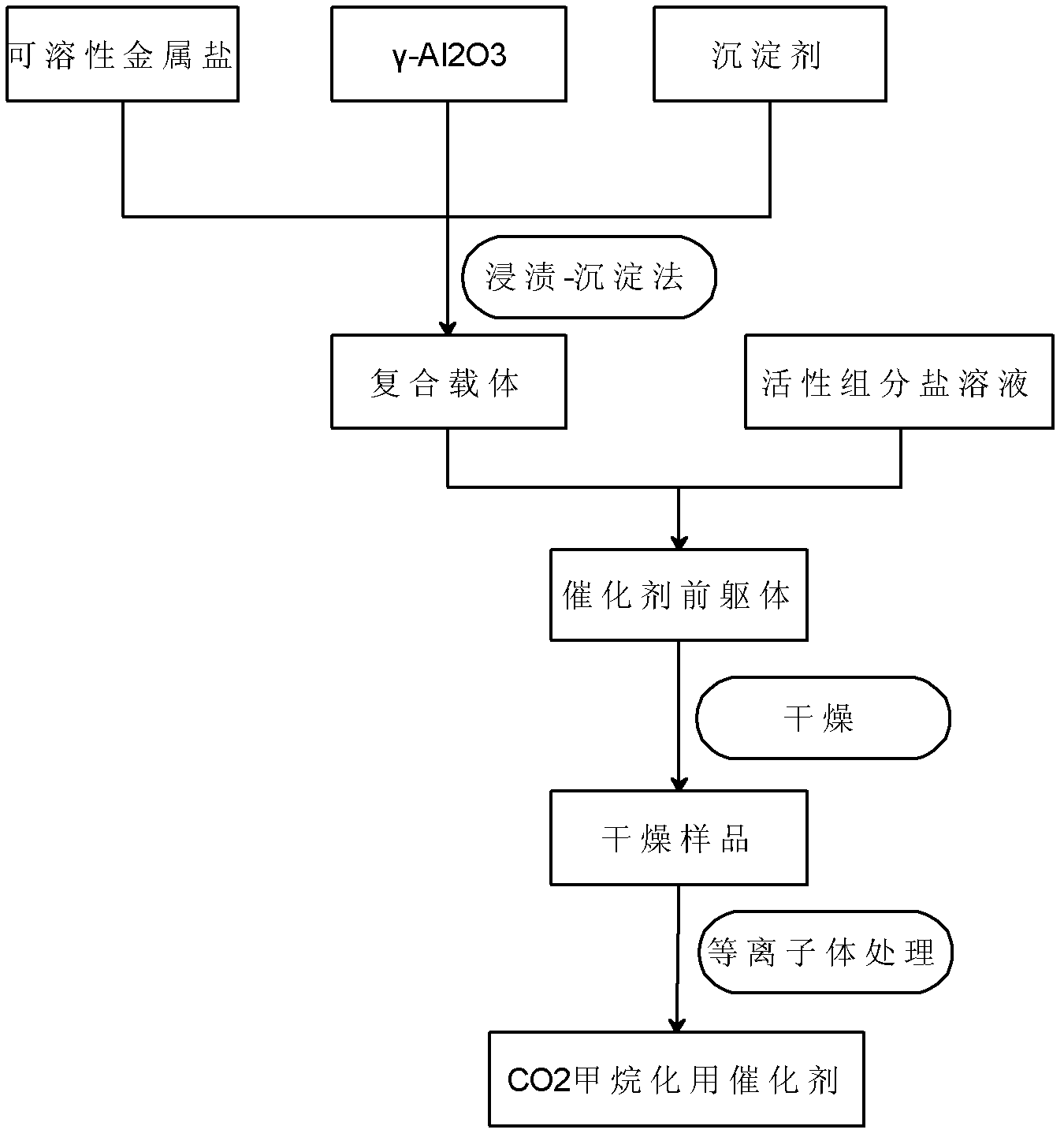
[0034] The concrete steps of the catalyst preparation method for carbon dioxide methanation of the present invention are:
[0035] 1) Prepare the composite support by impregnation-precipitation method: impregnate the salt solution corresponding to the water-soluble metal oxide in γ-Al by stirring at room temperature 2 o 3 On the carrier, the immersion time is 4-6 hours, so that the salt solution corresponding to the water-soluble metal oxide can completely enter the γ-Al 2 o 3 In the carrier; reintroduce precipitating agent to solution pH value is 8-10 (Ce(OH) when pH value ≥ 8 3 Precipitation begins to form. In an alkaline environment with a pH value of 8-10, it is easy to form small grains with better dispersion. If the pH value is too high, the particles will be agglomerated sharply). The purpose of adding a precipitant is to better The salt solution corresponding to the water-soluble metal oxide is uniformly loaded on the base carrier γ-Al 2 o 3 Surface: After the sol...
Embodiment 1
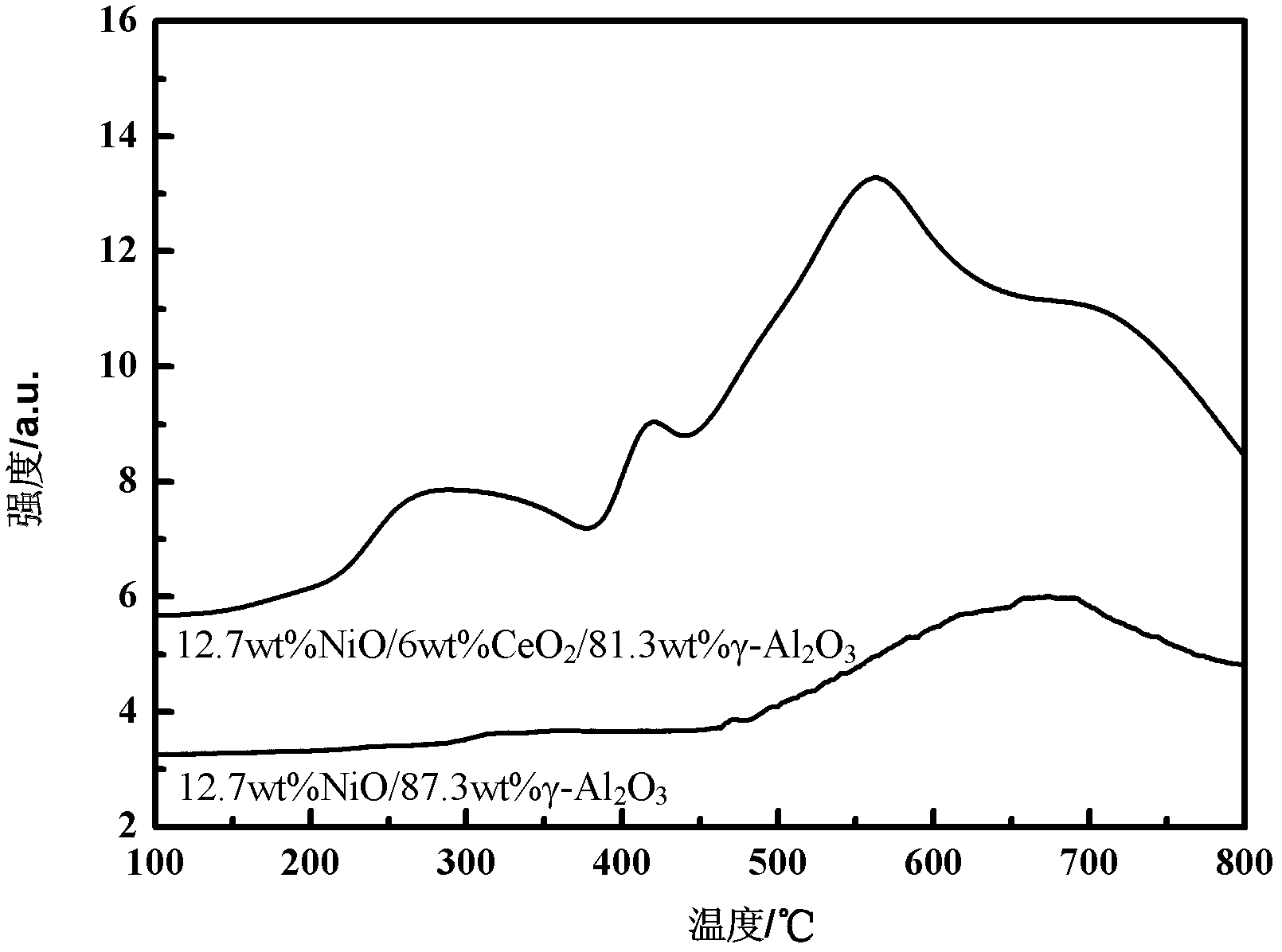
[0046] Example 1 12.7wt% NiO / 6.0wt% CeO 2 -81.3wt% γ-Al 2 o 3 Catalyst preparation
[0047] A First, CeO was prepared by impregnation-precipitation method 2 / γ-Al 2 o 3 Composite carrier: weigh 1.06g Ce(NO 3 ) 3 ·6H 2 Put O in a beaker, add 20ml of deionized water and stir to dissolve; weigh 4.65g of γ-Al 2 o 3 Place in the above solution, stir at room temperature for 60min, then slowly add the precipitating agent NH 3 ·H 2 O (0.8mol / L) to pH = 9, stirred for 2 hours to make the precipitate uniform, aged (aged means standing at room temperature) for 2 hours, filtered, washed, dried overnight at 120°C, and then roasted at 550°C for 5 hours. Get CeO 2 / γ-Al 2 o 3 Composite support, where CeO 2 The mass fraction of is 6.0wt%;
[0048] The preparation of B catalyst precursor: weigh 2.83g Ni(NO 3 ) 2 (Analytical pure, commercially available) is placed in a beaker, add 20ml of deionized water and stir to dissolve it; weigh 5g of CeO prepared in step A 2 / γ-Al 2 o...
Embodiment 2-11
[0052] Compared with Example 1, only the content of catalyst components or the types of nickel salt and water-soluble metal salt used are different, and other processes are the same as in Example 1 to obtain various finished catalysts. The catalyst compositions of Examples 2 to 11 and the nickel salts and water-soluble metal salts used are shown in Table 1.
[0053] Table 1 Catalyst Composition Table
[0054]
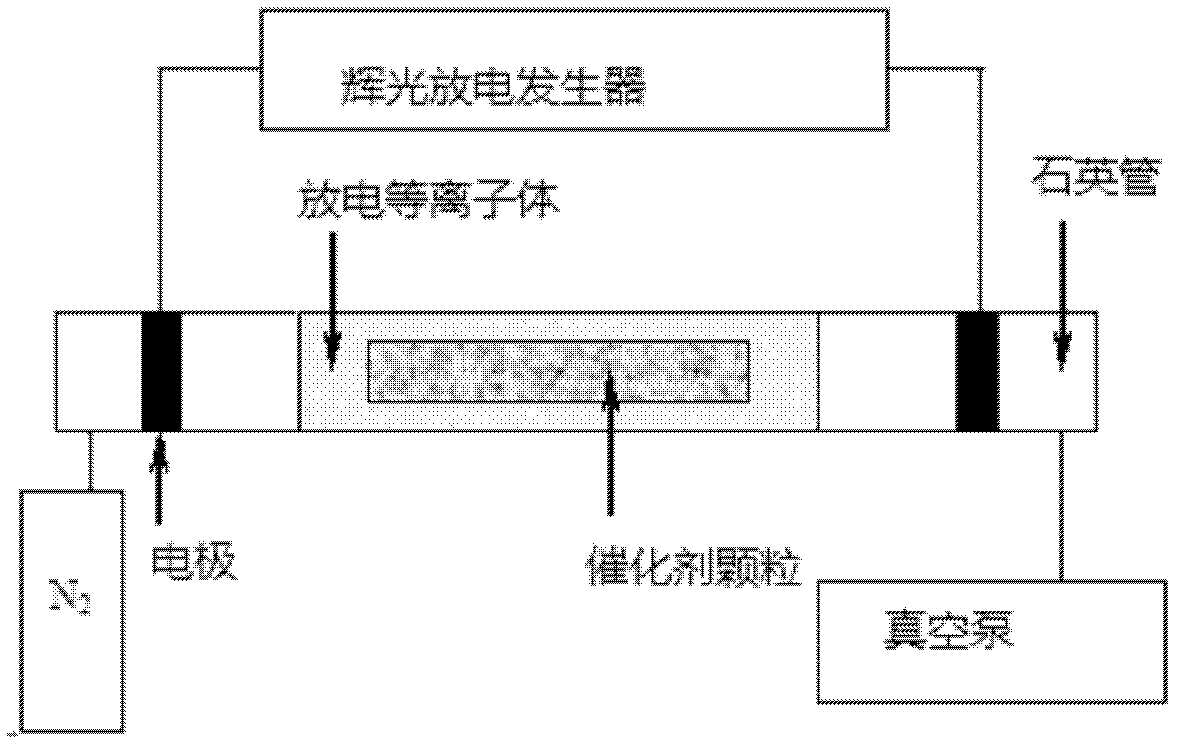
[0055] The catalyst obtained in Examples 1-11 was pressed into tablets and sieved to obtain 60-80 mesh catalyst particles, and 200 mg was filled in a fixed-bed reactor, and hydrogen was used for in-situ reduction. The reduction temperature was 450 ° C, and the reaction pressure was normal pressure. Raw material gas ratio is n(H 2 ):n(CO 2 )=4:1, gas volume space velocity 10000ml / h g cat , the investigation temperature range is 240-360°C, the tail gas composition is analyzed by TDX01 chromatographic column of GC-1690 gas chromatograph (TCD), and the data is recorde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com