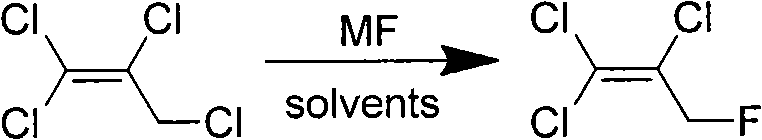
Synthesis method of 1,1,2-trichloro-3-fluoropropylene
A synthetic method and technology of fluoropropene, applied in 1 field, can solve the problems of high reaction temperature, low conversion rate and selectivity, and no reaction data given, and achieve the effect of high conversion rate and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 5.0g (0.028mol) of 1,1,2,3-tetrachloropropene and 30mL of diethylene glycol to a 50mL dry three-neck flask equipped with a magnetic stirrer, a thermometer, and a condensing device, start stirring, and heat up to 120 After ℃, add 8.1g (0.069mol) potassium fluoride to the reaction solution, react for 1 hour, the reaction solution is cooled to room temperature, filter the reaction solution to obtain 7.4g white solid, the filtrate is subtractively distilled, under vacuum 5kPa, collect 40~48 °C fractions to obtain 1,1,2-trichloro-3-fluoropropene, the conversion rate of 1,1,2,3-tetrachloropropene was 96.4%, the selection of 1,2-trichloro-3-fluoropropene sex 92.2%.
[0021] Product Structure Characterization:
[0022] bp: 135°C
[0023] MS: m / z 163.95 (M + )
[0024] 1 H-NMR (CDCl 3 , 500MHz) δ: 5.195(s, 1H), 5.101(s, 1H), J H-F =47Hz
[0025] 13 C-NMR (CDCl 3 , 500MHz) δ: 127.112-127.246 (d, 1C, J C-F =67Hz), 124.724-124.811 (d, 1C, J C-F =43.5Hz), 80.343-80.7...
Embodiment 2~4
[0028] The operation is basically the same as in Example 1, except that the reaction temperature and the reaction time are different, and the reaction results are shown in Table 1.
[0029] Table 1
[0030] Example Reaction temperature / ℃ Reaction time / h Conversion rates / % selectivity / % 1 110 1.5 96.4 92.2 2 120 1.0 98.7 90.0 3 100 4 98.9 93.1 4 90 10 70.1 94.5
Embodiment 5~9
[0032] The operation is basically the same as in Example 1, except that the solvent of Examples 5 to 9 is ethylene glycol, the reaction time is 4h, and the mol ratio of KF and 1,1,2,3-tetrachloropropene is respectively 1:1 , 1.5:1, 2:1, 2.5:1, 5:1, the reaction results are shown in Table 2.
[0033] Table 2
[0034] Example KF:TCP / (mol:mol) Conversion rates / % selectivity / % 5 1.0 65.3 92.1 6 1.5 85.7 92.4 7 2.0 95.4 92.2 8 2.5 98.2 91.8 9 5.0 99.2 92.5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com