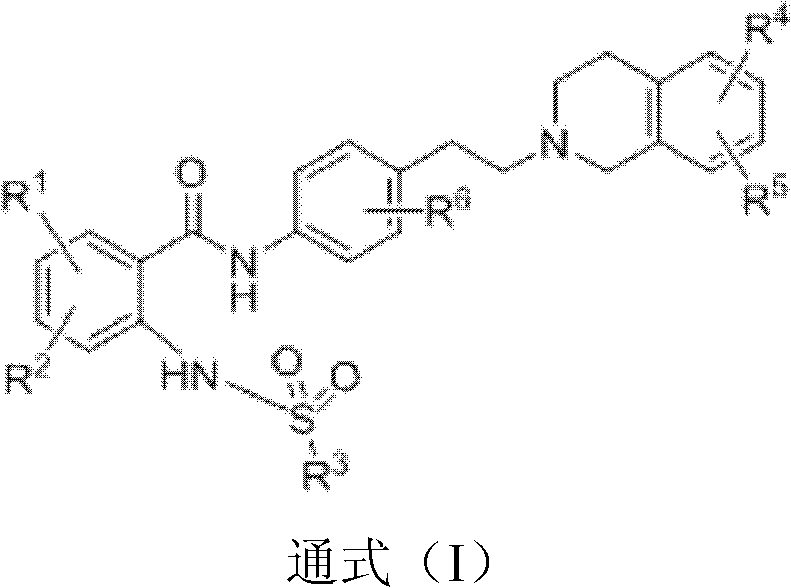
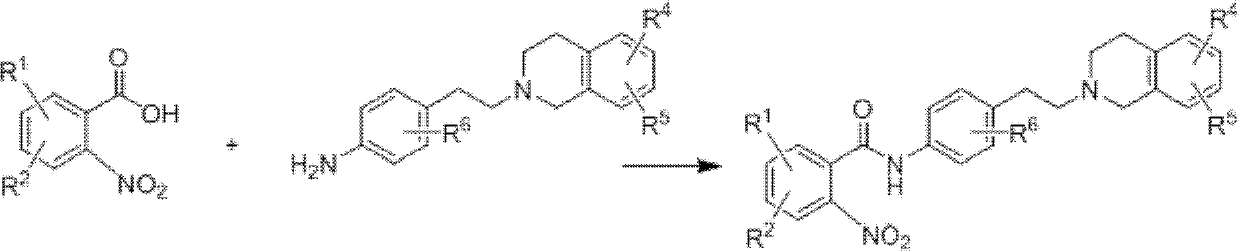
O-aminobenzoic acid sulfonylation derivative as well as preparation method and application thereof
A technology of anthranilic acid sulfonylated derivatives and aminobenzoic acid sulfonylated derivatives, which is applied in the field of medicinal chemistry and can solve the problems of limited application, lack of specificity and affinity of P-gp, affecting P-glycoprotein efflux and other issues, to achieve the effect of high selectivity and inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] p-methoxyphenyl (2-{4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-phenylamino Chemical Synthesis of Formyl}-phenyl)sulfonamide (S1)
[0039] (1) Drop 3.3404g o-nitrobenzoic acid (20mmol) into the reactor, add 100mL methylene chloride, after stirring and dissolving, drop 2.7032g 1-hydroxybenzotriazole (20mmol) and 7.5807g benzene under ice bath Triazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (20mmol), 7.0mL N,N-diisopropylethylamine (20mmol), stirred at 0°C for 10 minutes , then add 4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-aniline, react at room temperature for 24 hours, after the reaction is complete The mixture was diluted with an appropriate amount of dichloromethane, washed with saturated sodium bicarbonate and water for 2 to 3 times, added a small amount of magnesium sulfate solid particles to dry, filtered, concentrated under reduced pressure, evaporated the solvent, and separated by silica gel chromatography to obtain P...
Embodiment 2
[0046] Benzyl (2-{4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-phenylcarbamoyl}- Chemical Synthesis of Phenyl)sulfonamide (S2)
[0047] (1) Put 3.3404g o-nitrobenzoic acid (20mmol) into the reactor, add 100mL dichloromethane and stir to dissolve, then drop 2.7104g 1-hydroxybenzotriazole (20mmol) and 7.5803g benzotriazole under ice bath Triazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (20mmol), 7.0mL N,N-diisopropylethylamine (20mmol), stirred at 0°C for 10 minutes, Then add 4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-aniline, react at room temperature for 24 hours, after the reaction is complete The mixture was diluted with an appropriate amount of dichloromethane, washed successively with saturated sodium bicarbonate and water for 2 to 3 times, added a small amount of magnesium sulfate solid particles to dry, filtered, concentrated under reduced pressure, evaporated the solvent, and separated by silica gel chromatography to obtain pure...
Embodiment 3
[0054] p-methylphenyl (2-{4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-phenylaminomethyl Chemical Synthesis of Acyl}-phenyl)sulfonamide (S3)
[0055] (1) Put 3.3407g o-nitrobenzoic acid (20mmol) into the reactor, add 100mL dichloromethane and stir to dissolve, then drop 2.7023g 1-hydroxybenzotriazole (20mmol) and 7.5811g benzotriazole under ice bath Triazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (20mmol), 7.0mL N,N-diisopropylethylamine (20mmol), stirred at 0°C for 10 minutes, Then add 4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-aniline, react at room temperature for 24 hours, after the reaction is complete The mixture was diluted with an appropriate amount of dichloromethane, washed successively with saturated sodium bicarbonate and water for 2 to 3 times, added a small amount of magnesium sulfate solid particles to dry, filtered, concentrated under reduced pressure, evaporated the solvent, and separated by silica gel chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com