Tanshinone derivatives, medicine compositions thereof, and purposes thereof in medicine
A technology of tanshinone and derivatives, applied in the field of tanshinone compounds, can solve the problems of no application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The specific experimental method of the present invention is as follows: using tanshinone IIA and cryptotanshinone separated from traditional Chinese medicine Danshen as starting materials, synthesizing tanshinone derivatives represented by general formula (I). Specifically, tanshinone IIA and cryptotanshinone are starting materials for the preparation of such compounds, and tanshinone IIA (T1) and cryptotanshinone (T2) can be isolated from the ethanol extract of traditional Chinese medicine Danshen.
[0053] The structural formulas of tanshinone IIA and cryptotanshinone are:
[0054]
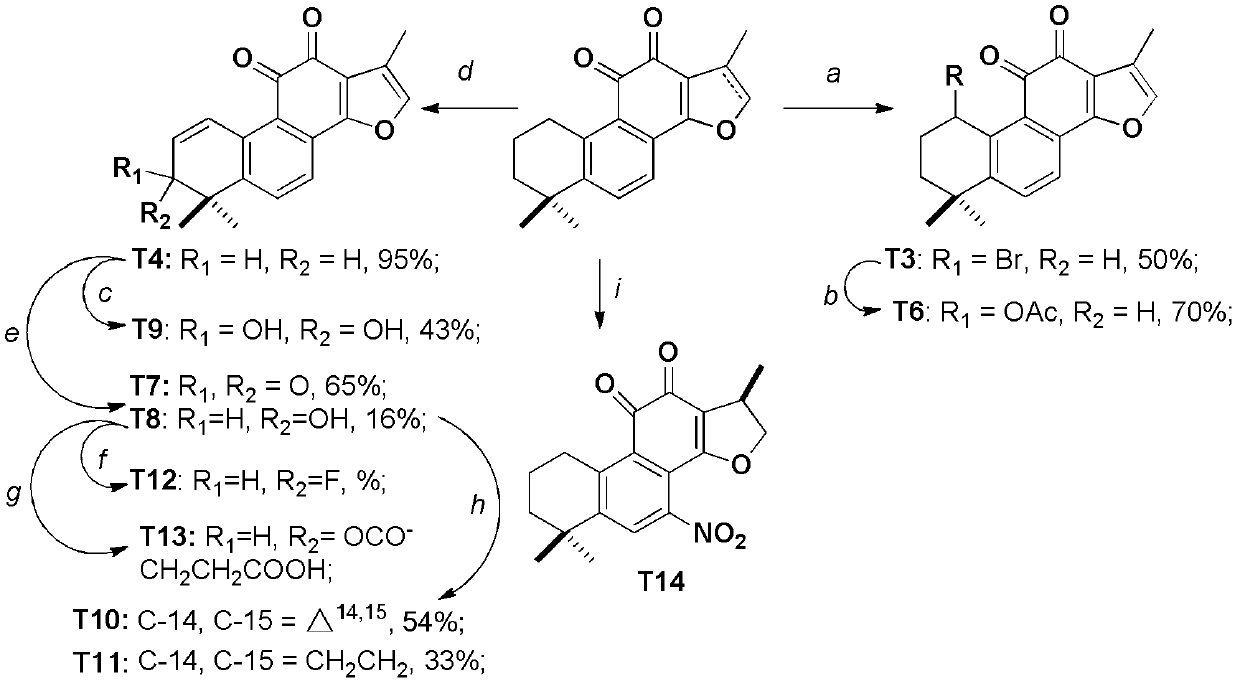
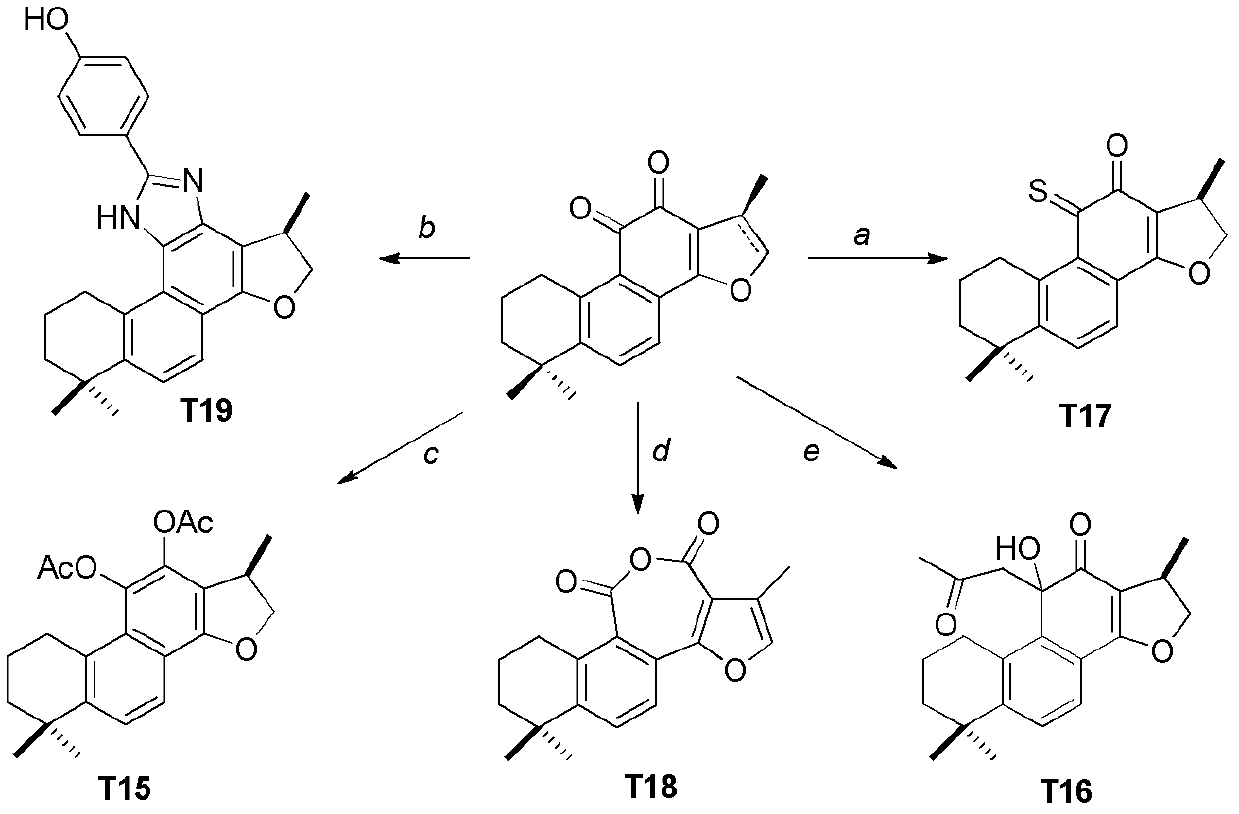
[0055] The tanshinone compound shown in general formula (I) can be obtained through 1-4 step reaction by tanshinone IIA and cryptotanshinone through the reaction scheme among the embodiment 1 (see reaction scheme, figure 2 , 3 , 4).
[0056] figure 2 It is a reaction flow diagram for compounds derived from tanshinone IIA and cryptotanshinone A ring and B ring; reagents and reacti...
Embodiment 2
[0063] Preparation of compounds T3, T4, T5:
[0064]
[0065] To the CCl of compound T1 (29 mg, 0.1 mmol) 4 (5mL) solution, add NBS (27mg, 0.15mmol) and benzoyl peroxide (36mg, 0.15mmol) successively. After the addition, take a breath (N 2 ), and then reacted for 3 hours at room temperature. After the reaction is complete, add DCM to dilute, then use saturated Na 2 SO 3 Wash with aqueous solution, extract the aqueous phase with DCM three times (5mL×3), combine the organic phases, wash with saturated aqueous sodium chloride, dry over anhydrous sodium sulfate and concentrate, then column chromatography (petroleum ether:ethyl acetate=15:1), Compound T3 (15 mg, 50%) was obtained. The structural analysis data of compound T3 are as follows:
[0066] ESI-MS was measured on a shimadzu LCMS-2010EV mass spectrometer; 1D and 2D NMR were measured on a BrukerAM-300 and DRX-500 nuclear magnetic resonance instrument, the deuterated reagent used was produced by Sigma Aldrich, TMS was...
Embodiment 3
[0070] Preparation of Compound T6:
[0071]
[0072] To a solution of compound T3 (18 mg, 0.05 mmol) in DMF (1.0 mL), NaOAc (16 mg, 0.2 mmol) and TBAI (3 mg) were added sequentially. After the addition, the reaction was carried out at room temperature for 12 hours. After completion of the reaction, dilute with water (10 mL), then extract three times with EtOAc (8 mL × 3), combine the organic phases, wash with water and saturated aqueous sodium chloride successively, dry over anhydrous sodium sulfate, concentrate and column chromatography (petroleum ether: Ethyl acetate=6:1) to obtain compound T6 (12 mg, 70%).
[0073] Compound T6:: red solid, 1 H-NMR (500MHz, CDCl 3 )δ7.73(d, J=10Hz, 1H), 7.72(s, 1H), 6.41(s, 1H), 2.21(s, 3H), 2.02(s, 3H), 1.90-1.98(m, 2H) , 1.60(m, 4H), 1.40(s, 3H), 1.28(s, 3H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com