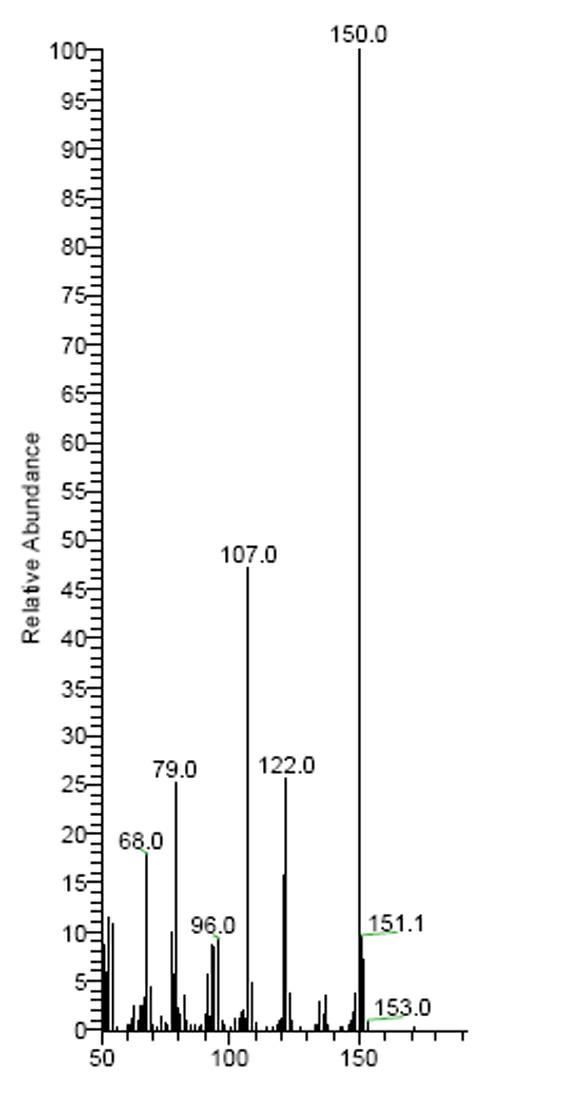
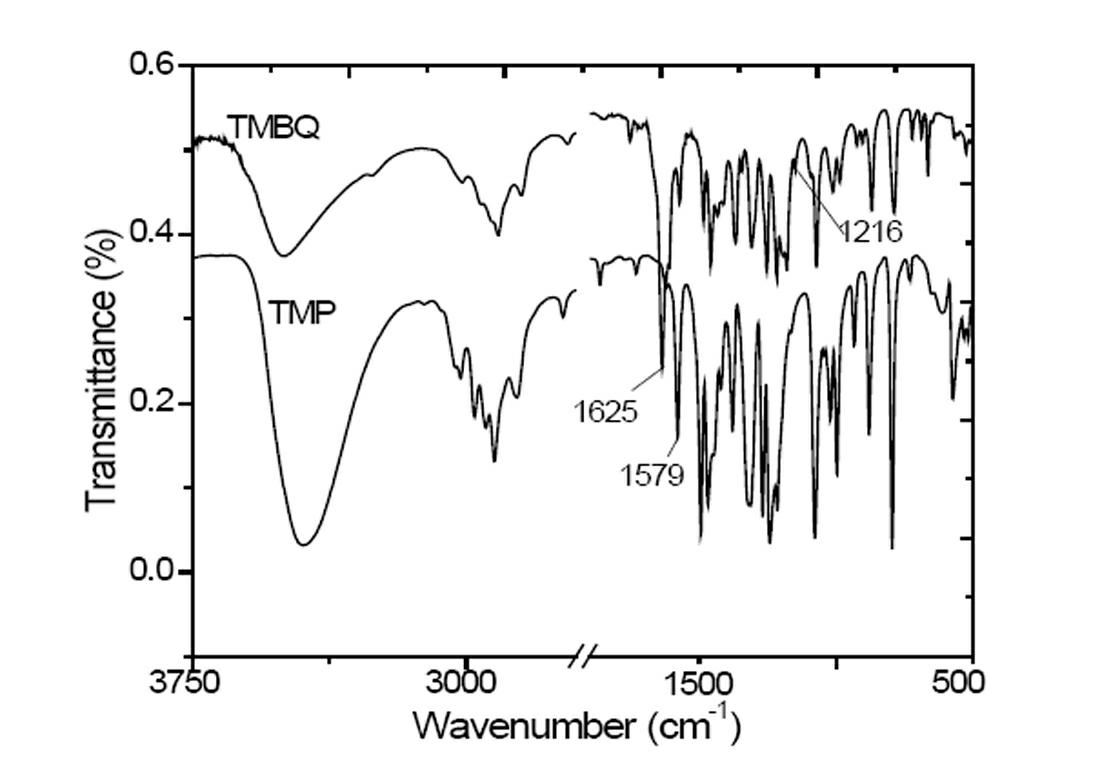
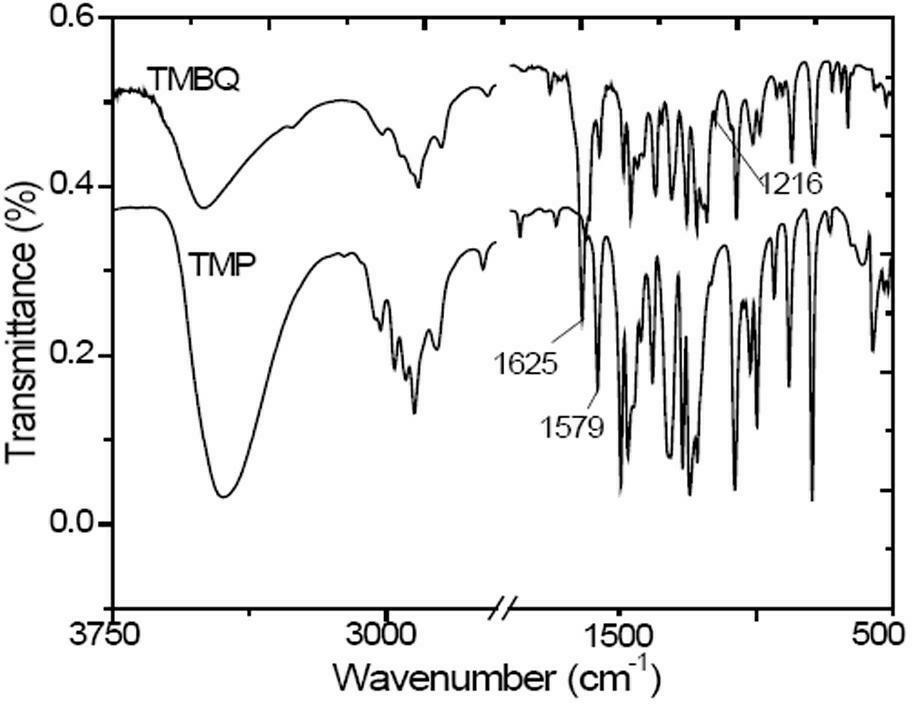
Preparation method of 2, 3, 5-trimethyl benzoquinone (TMBQ)
A technology of trimethylbenzoquinone and triethylamine hydrochloride, applied in 2 fields, to achieve the effects of mild reaction conditions, promotion of catalytic oxidation, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation method of 2,3,5-trimethylbenzoquinone provided in this embodiment comprises the following steps:
[0018] (1) the triethylamine hydrochloride of 0.0015mol and the FeCl of 0.00015mol 3 Mix, stir at 35 °C for 1 h, then add 0.0030 mol of CuCl 2 , continue to stir for 1 hour at 35 ° C, and finally add 10 drops of concentrated HCl, continue to stir for 1 hour to prepare a composite catalyst;
[0019] (2) Dissolve 0.015mol of TMP in 25mL of acetic acid, mix well, and prepare a TMP acetic acid solution whose concentration is 0.6mol / L;
[0020] (3) Add the composite catalyst prepared in step (1) into the TMP acetic acid solution prepared in step (2), stir vigorously to make it evenly mixed, then heat to reflux under vigorous stirring to obtain reaction system A, and then start the peristaltic Pump, add hydrogen peroxide aqueous solution dropwise to reaction system A, the hydrogen peroxide aqueous solution contains 0.45mol of H 2 o 2 , the volume percentage c...
Embodiment 2
[0023] The preparation method of 2,3,5-trimethylbenzoquinone provided in this embodiment comprises the following steps:
[0024] (1) the triethylamine hydrochloride of 0.0015mol and the FeCl of 0.00015mol 3 Mix and stir at 35 °C for 1 h, then add 0.0015 mol of CuCl 2 , continue stirring at 35°C for 1 hour to obtain a composite catalyst;
[0025] (2) The TMP of 0.015mol and the benzoic acid of 0.0048mol are dissolved in 50mL ethanol, mix uniformly, make solution B, the concentration of TMP in solution B is 0.3mol / L;
[0026](3) Add the composite catalyst prepared in step (1) to the solution B prepared in step (2), stir vigorously to make it evenly mixed, then heat to reflux under vigorous stirring to obtain reaction system A, and then turn on the peristaltic pump , add dropwise hydrogen peroxide aqueous solution to the reaction system A, the hydrogen peroxide aqueous solution contains 0.45mol of H 2 o 2 , the volume percent concentration of the hydrogen peroxide aqueous sol...
Embodiment 3
[0029] The preparation method of 2,3,5-trimethylbenzoquinone provided in this embodiment comprises the following steps:
[0030] (1) the triethylamine hydrochloride of 0.0015mol and the FeCl of 0.00015mol 3 Mix and stir at 35 °C for 1 h, then add 0.0015 mol of CoCl 2 , continue stirring for 1 hour at 35°C to obtain a composite catalyst;
[0031] (2) 0.015mol of TMP was dissolved in 10mL of ethyl acetate, and mixed uniformly to obtain an ethyl acetate solution of TMP;
[0032] (3) Add the composite catalyst prepared in step (1) into the ethyl acetate solution of TMP prepared in step (2), stir vigorously to make it evenly mixed, then heat to reflux under vigorous stirring to obtain reaction system A, Afterwards, the peristaltic pump was turned on, and an aqueous hydrogen peroxide solution was added dropwise to the reaction system A, which contained 0.50mol of H 2 o 2 , the volume percent concentration of the hydrogen peroxide aqueous solution is 20%. After the hydrogen perox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com