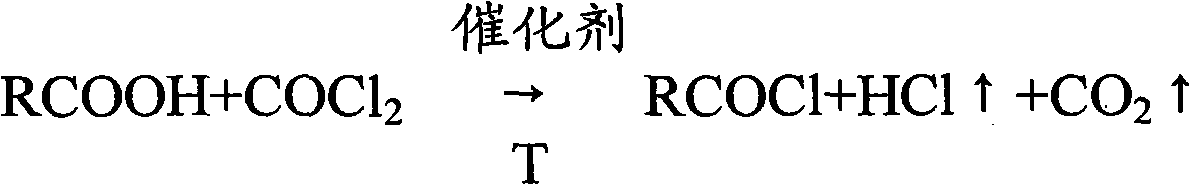Method for producing stearoyl chloride and homologs thereof by liquid-phase phosgenation
A technology of stearoyl chloride and phosgenation, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as difficult absorption, low reaction intensity, slow reaction speed, etc., to improve reaction driving force, The effect of improving the update speed and reducing the response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
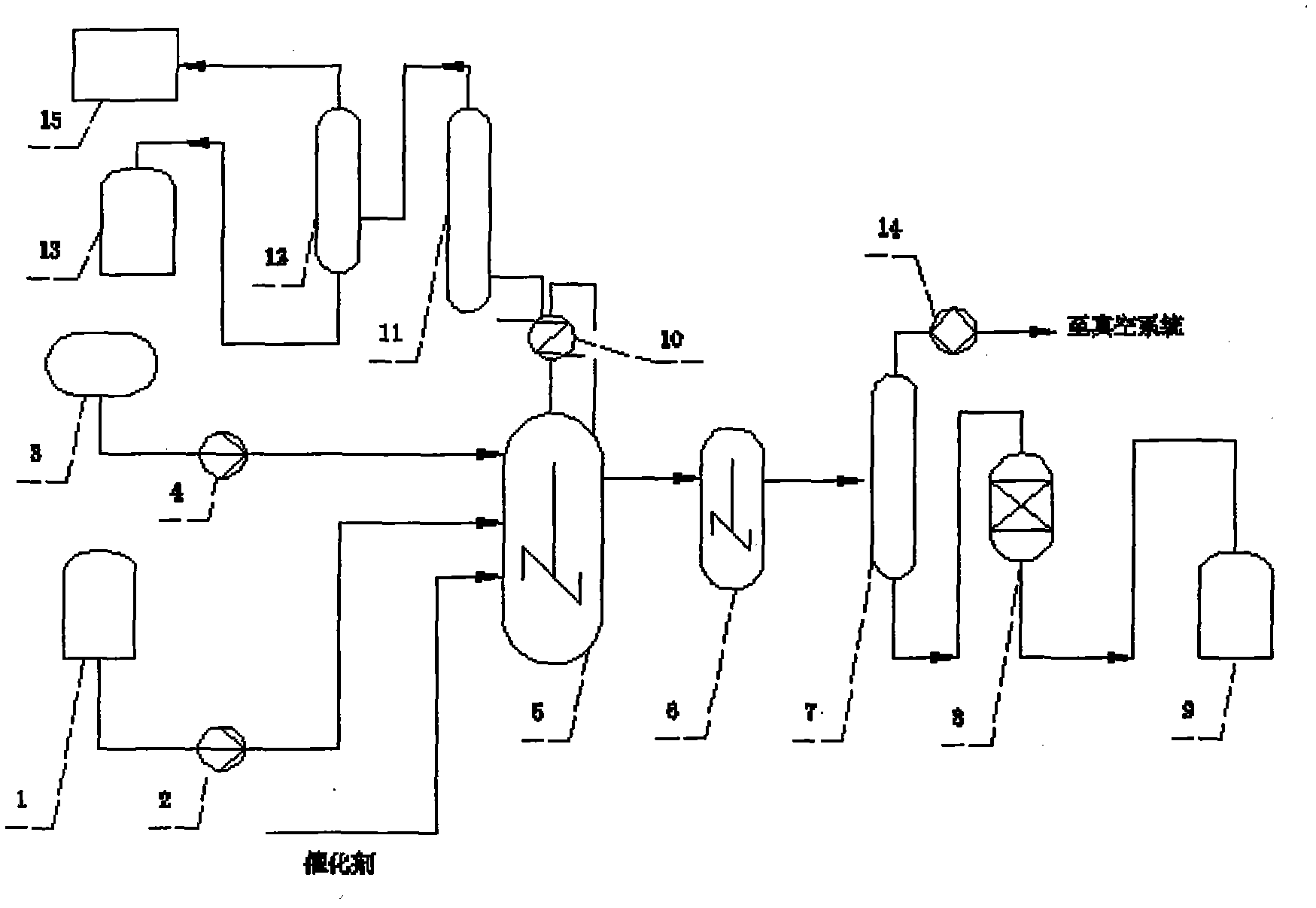
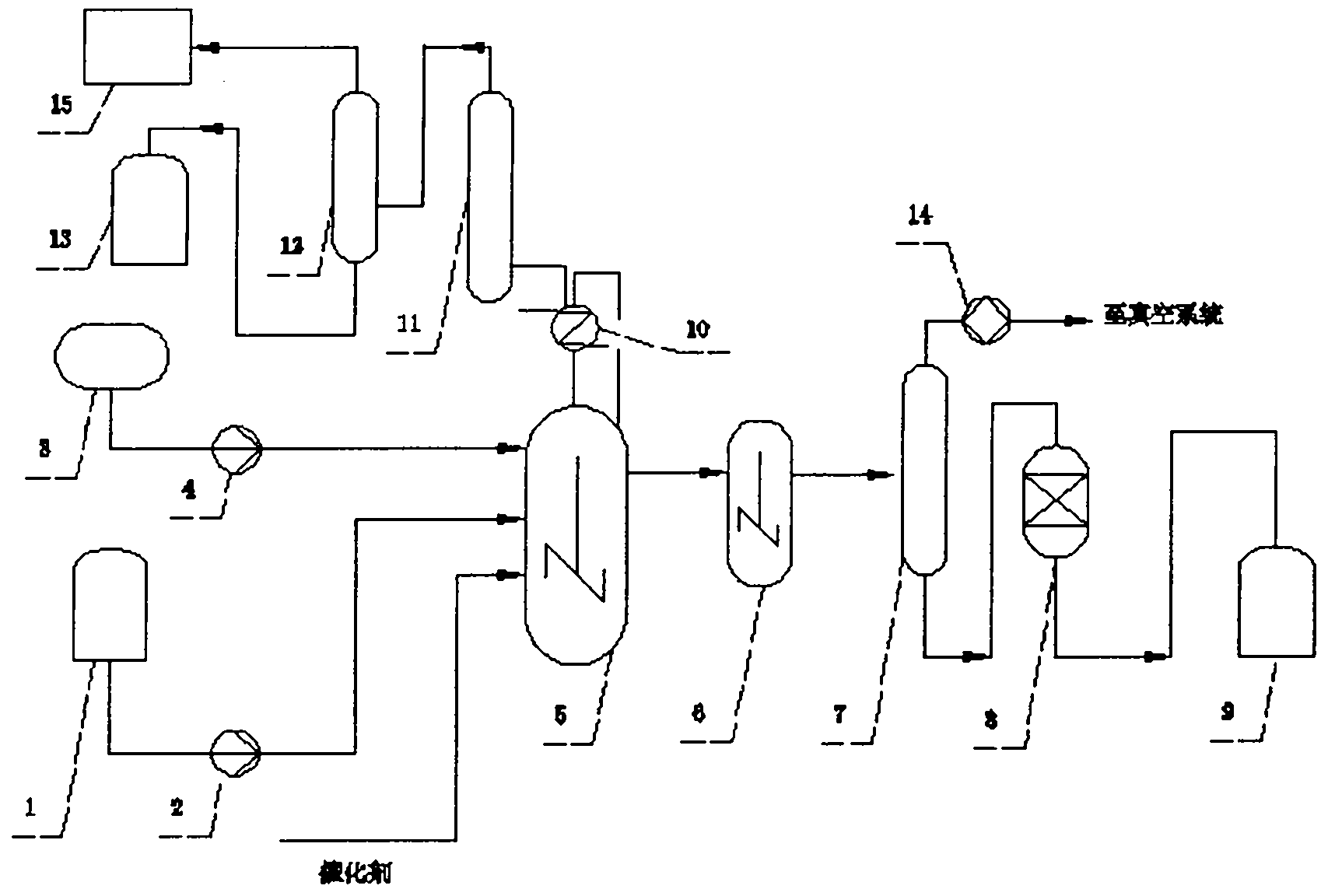
[0036] Example 1, see figure 1 , a method for producing stearyl chloride and its homologues by liquid-phase phosgenation, characterized in that: continuous operation, raw material A, catalyzer, phosgene are added to the main photochemical reactor 5 at the same time, through the main photochemical reaction Kettle 5, photochemical reaction maturation kettle 6 reaction and negative pressure dephosphorization tower 7 dephosphorization, stearoyl chloride product decolorization device 8 decolorizes and continuously produces stearoyl chloride products, and the raw material A is stearic acid or its homologue One or two to three mixtures of its homologues, the specific steps are as follows:
[0037] a, the raw material A in the raw material A storage tank 1 is added to the main photochemical reaction kettle 5 through the delivery pump 2, and at the same time, the liquid phase phosgene in the liquid phosgene storage tank 3 is added to the main photochemical reaction kettle 5 through the...
Embodiment 2
[0053] Example 2, see figure 1 , step is with embodiment 1, and concrete process parameter is:
[0054] φ450 mm stirring main photochemical reactor, reactor height 650 mm, stirrer speed 450 rpm; φ300 mm stirred photochemical reaction maturation tank, reactor height 450 mm, stirrer speed 350 rpm. The amount of molten stearic acid added is: 45 kg / hour, the amount of dimethylformamide added is 0.6 kg / hour, and the amount of phosgene added is 18 kg / hour, which is continuously added to the reactor for reaction, and the temperature of the reactor is controlled 90~95℃, the reaction pressure is controlled at 0.8-1.0MPa, the temperature of the tank top condenser is controlled at 8-12℃, the non-condensable tail gas of the tank top condenser passes through the phosgene rectification tower and the HCl absorption tower, and then enters the alkali liquid destruction tower for destruction treatment ; Negative pressure dephosphorization tower control temperature 80-110 ℃, negative pressure c...
Embodiment 3
[0055] Example 3, see figure 1 :
[0056] φ450 mm stirring main photochemical reactor, reactor height 650 mm, stirrer speed 450 rpm; φ300 mm stirred photochemical reaction maturation tank, reactor height 450 mm, stirrer speed 350 rpm. Molten state stearic acid, palmitic acid mixture (stearic acid 35%, palmitic acid 65%) charging amount is: 50 kilograms / hour, strongly acidic cationic resin dimethylformamide adsorption solidified product adding amount is 3.8 kg, the amount of phosgene added is 23 kg / hour, and it is continuously added to the reactor for reaction. The non-condensable tail gas of the top condenser passes through the phosgene rectification tower and the HCl absorption tower, and then enters the lye destruction tower for destruction; the negative pressure dephosphorization tower controls the temperature at 70-90°C, and the negative pressure is controlled at 8KPa(a), and then goes through the decolorization bed. After 3 hours of stable reaction and feeding, start sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com