New preparation method of 5-nitro-salicylaldehyde
A technology of nitrosalicylaldehyde and salicylaldehyde, which is applied in the preparation of nitro compounds, organic chemistry and other directions, can solve the problems such as unfavorable large-scale production of 5-nitrosalicylaldehyde, difficulty in separation and purification, low reaction rate and the like , to achieve the effect of being beneficial to large-scale synthesis, increasing the reaction rate and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
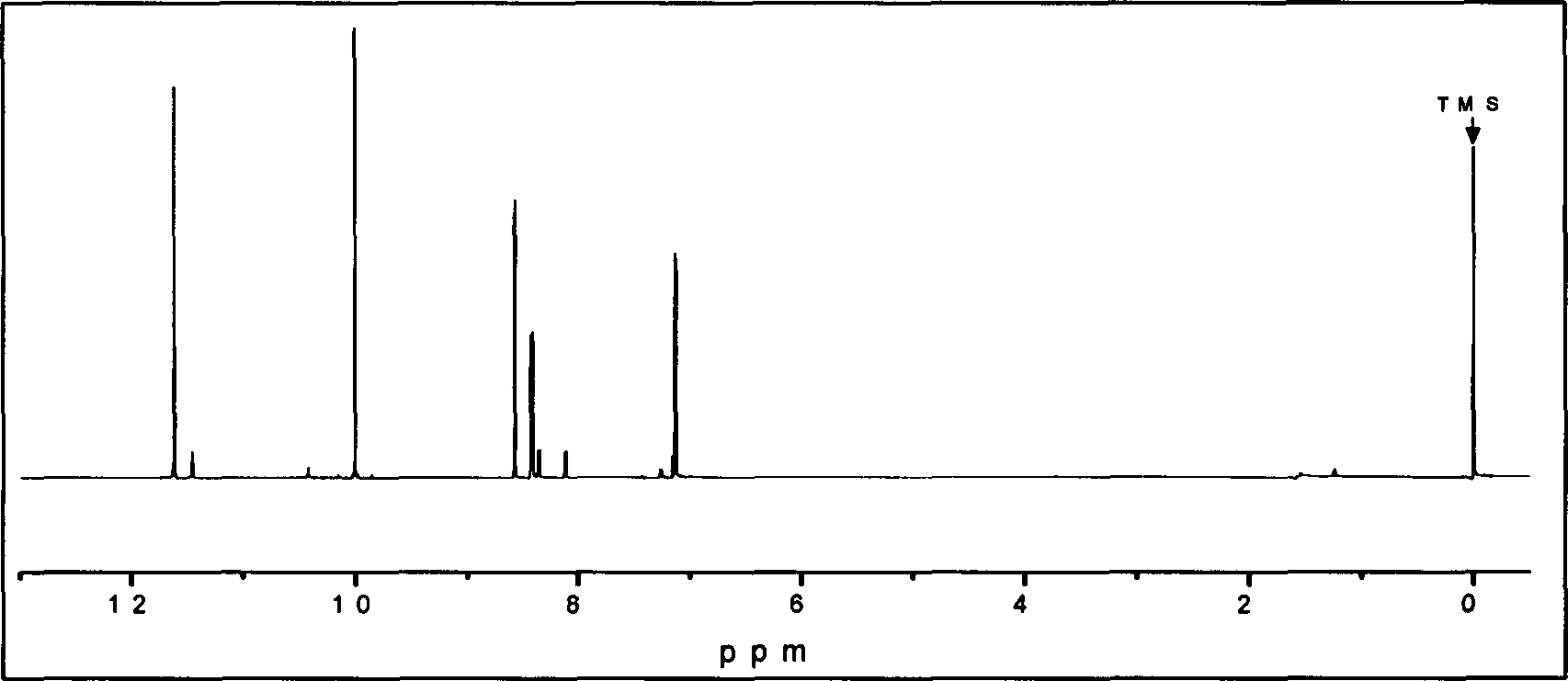
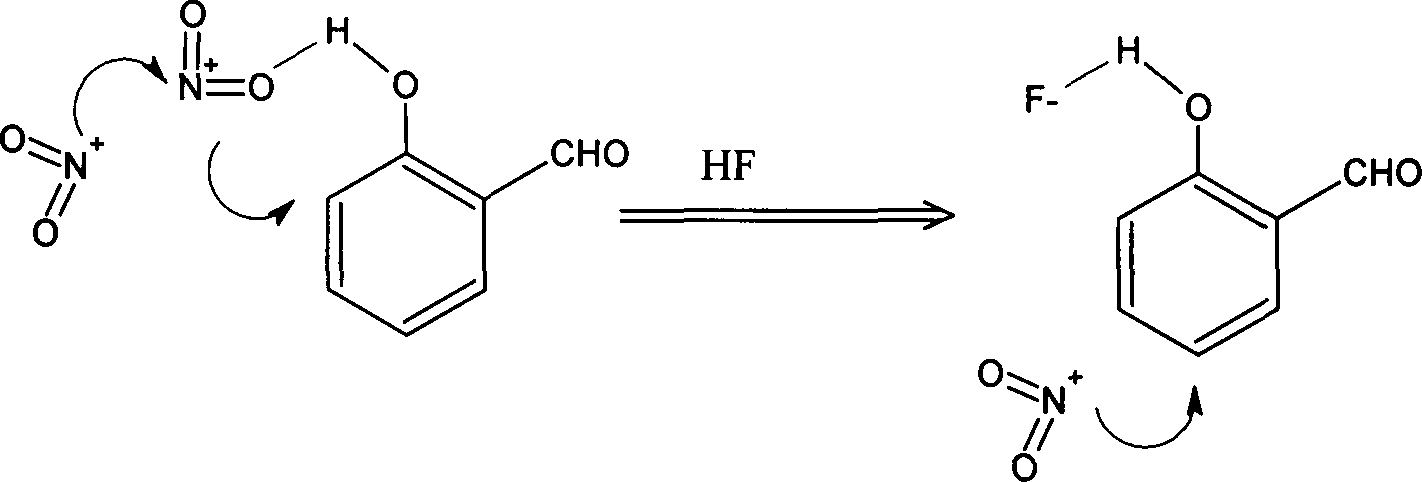
[0022] Measure 100ml of glacial acetic acid, 12.5g of hydrofluoric acid, 25.9g of acetic anhydride, and 25.7g of salicylaldehyde into a 250ml three-neck flask in turn, and keep stirring in an ice-water bath to cool below 5°C. Put 16.6g of fuming nitric acid in a constant pressure dropping funnel, control the temperature within the range of 5-10°C, and evenly drop the fuming nitric acid into the three-necked flask within 2.5 hours. Then control the temperature at 8-10°C to react for 1 hour, then raise the temperature to 15-20°C to continue the reaction for 1 hour. While hot, pour the reaction solution into a mixture of 500 g of crushed ice and water, stir evenly, and let stand for 5 hours (or stand overnight). Suction filtration, washing and drying gave 28.3 g of yellow powder solid. After water extraction, 14.8 g of light yellow 5-nitrosalicylaldehyde powder was obtained with a yield of 52%.
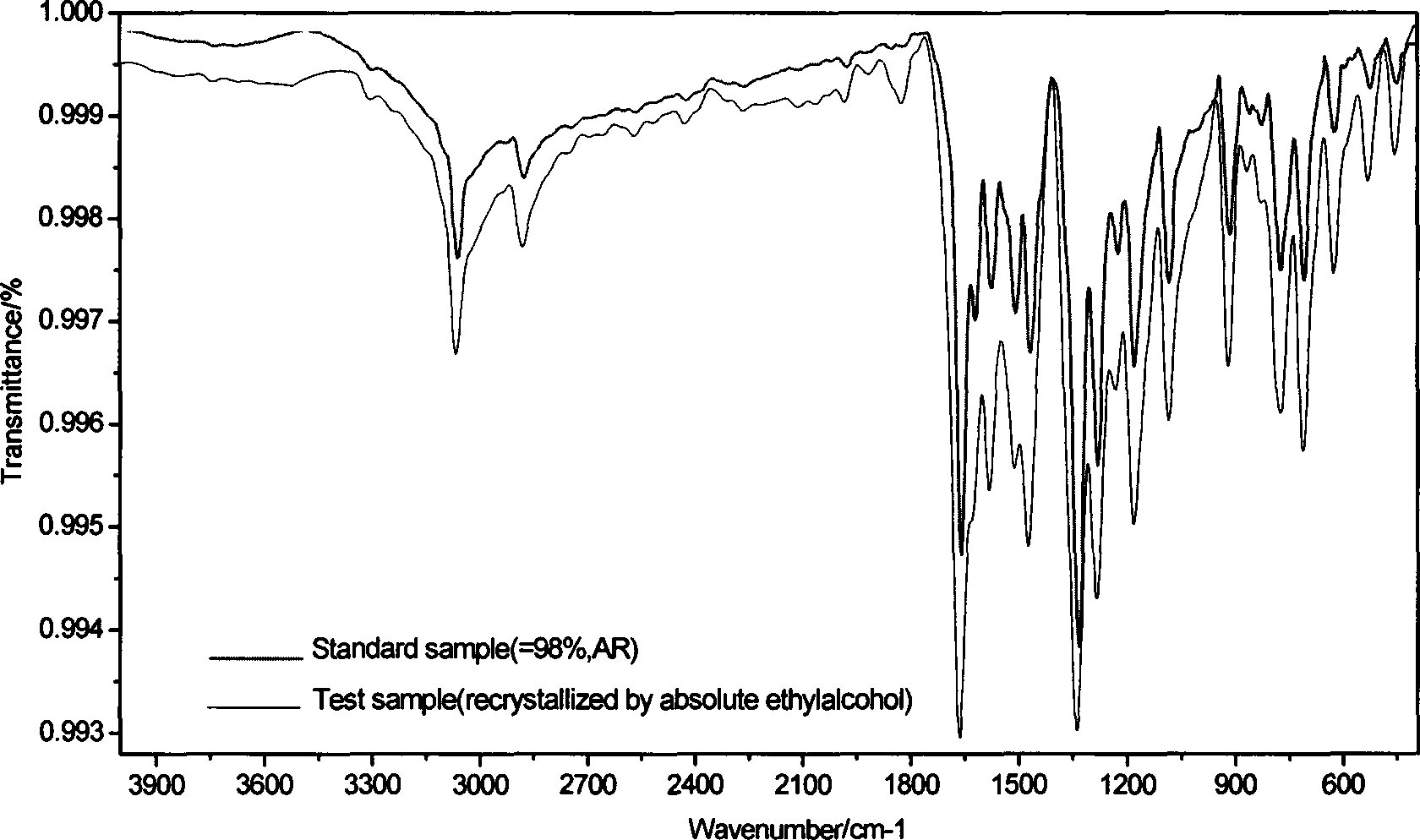
[0023] Infrared spectrum (KBr, cm -1 ): 3069cm -1 ;2883cm -1 ;1664cm -1 ;1625,...
Embodiment 2
[0026] Measure 100ml of glacial acetic acid, 12.5g of hydrofluoric acid, 25.9g of acetic anhydride, and 25.7g of salicylaldehyde into a 250ml three-neck flask in turn, and keep stirring in an ice-water bath to cool below 5°C. Put 16.6g of fuming nitric acid in a constant pressure dropping funnel, control the temperature in the range of 5 to 102, and evenly drop the fuming nitric acid into the three-necked flask within 2.5 hours. Then control the temperature at 8-10°C to react for 1 hour, then raise the temperature to 15-20°C to continue the reaction for 1 hour. While hot, pour the reaction solution into 500g of crushed ice and water mixture, stir evenly, and let stand for 5h (or stand overnight). Suction filtration, washing and drying gave 25.5 g of yellow powder solid. After water extraction, 12.7 g of light yellow 5-nitrosalicylaldehyde powder was obtained with a yield of 50%.
[0027] Infrared spectrum (KBr, cm -1 ): 3070cm -1 ;2885cm -1 ;1657cm -1 ;1614, 1578, 1447cm...
Embodiment 3
[0030] Measure 100ml of glacial acetic acid, 5.0g of hydrofluoric acid, 25.9g of acetic anhydride, and 25.7g of salicylaldehyde into a 250ml three-neck flask in turn, and keep stirring in an ice-water bath to cool below 5°C. Put 16.6g of fuming nitric acid in a constant pressure dropping funnel, control the temperature within the range of 5-10°C, and evenly drop the fuming nitric acid into the three-necked flask within 2.5 hours. Then control the temperature at 8-10°C to react for 1 hour, then raise the temperature to 15-20°C to continue the reaction for 1 hour. While hot, pour the reaction solution into 500g of crushed ice and water mixture, stir evenly, and let stand for 5h (or stand overnight). Suction filtration, washing and drying gave 32.0 g of yellow powder solid. After water extraction, 12.6 g of light yellow 5-nitrosalicylaldehyde powder was obtained with a yield of 39%. The reason for the substantial reduction in the yield of this synthesis is that the amount of hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com