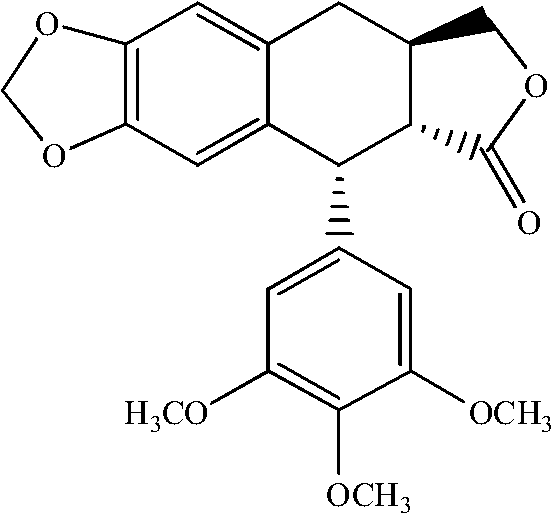
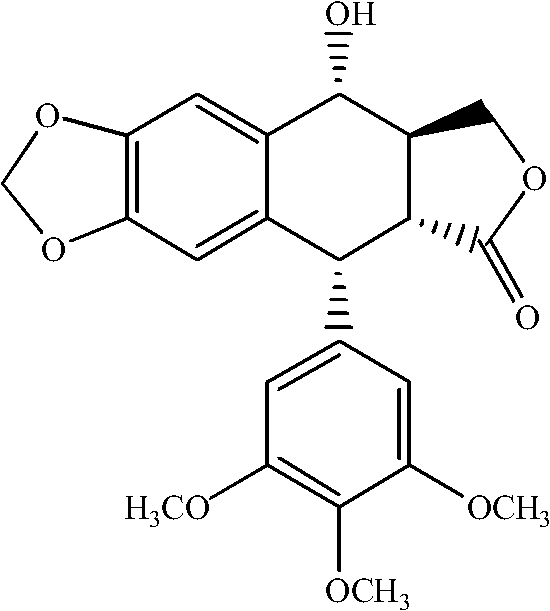
Manufacturing method for deoxypodophyllotoxin
A technology of deoxypodophyllotoxin and podophyllotoxin, which is applied in the field of natural medicine and chemical medicine, can solve the problems of high energy consumption of deoxypodophyllotoxin, potential safety hazards, increased funds, etc., and is suitable for industrial production and increases risks Coefficients, preparation of simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 1.0g podophyllotoxin and 0.91g NaBH to a 50ml single-neck bottle 4 , then add 25ml of anhydrous tetrahydrofuran; slowly add 15ml of CF 3 After the addition of COOH is complete, the temperature is raised to 70°C, and the reaction is completed for about 20 minutes. The reaction solution was poured into 100 ml of water, extracted three times with 10 ml of ethyl acetate, and the organic layer was separated. The organic layer was washed with 50 ml of saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to obtain the crude deoxypodophyllotoxin, which was then recrystallized from a mixed solvent of ethyl acetate:methanol=1:6 to obtain the pure deoxypodophyllotoxin with a purity of 99.2% and a yield of 80%. White crystalline powder, mp 165~167℃;
[0024] UV: In methanol solution, λmax 292.1, 204.3nm is the characteristic absorption of the B band and K band of the substituted benzene ring;
[0025] IR: 1589, 1506, 1483c...
Embodiment 2
[0029] Add 1.0g podophyllotoxin, 1.0g NaBH to a 50ml single-neck bottle 4 , then add 25ml anhydrous tetrahydrofuran; slowly add 20ml CF 3 COOH, after the dropwise addition was completed, the temperature was raised to 50°C, and the reaction was completed for about 2 hours. The reaction solution was poured into 100 ml of water, extracted three times with 10 ml of ethyl acetate, and the organic layer was separated. The organic layer was washed with 50 ml of saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to obtain crude deoxypodophyllotoxin, and then recrystallized from a mixed solvent of ethyl acetate:methanol=1:10 to obtain pure deoxypodophyllotoxin with a purity of 98.2%, and the yield was 82%.
Embodiment 3
[0031] Add 1.0g podophyllotoxin, 1.20g NaBH to a 50ml single-neck bottle 4 , then add 25ml anhydrous tetrahydrofuran; slowly add 25ml CF 3COOH, after the dropwise addition is complete, react at room temperature, react for about 72 hours, and the reaction is complete. The reaction solution was poured into 100 ml of water, extracted three times with 10 ml of ethyl acetate, and the organic layer was separated. The organic layer was washed with 50 ml of saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to obtain the crude deoxypodophyllotoxin, which was then recrystallized from a mixed solvent of ethyl acetate:methanol=1:4 to obtain the pure deoxypodophyllotoxin with a purity of 99.1% and a yield of 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com