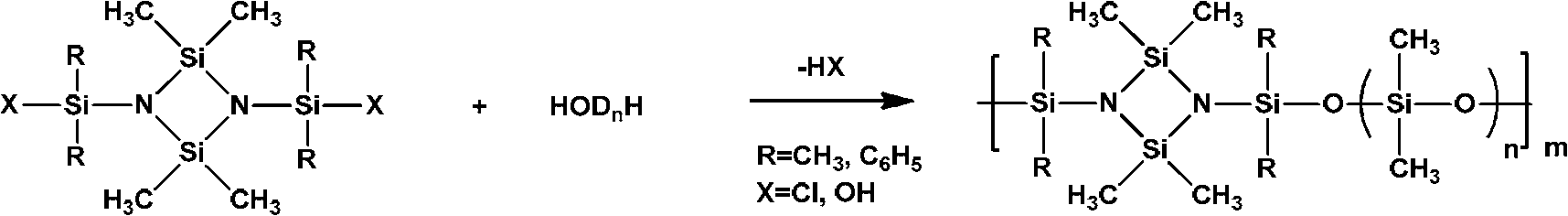Heat-resisting poly (silazane-siloxane) polymer and preparation method thereof
A technology of high temperature resistance and silazane, applied in the field of high temperature resistance copolymer and its preparation, can solve the problems of harsh synthesis and purification conditions and low yield, achieve excellent thermal stability and thermal oxidation stability, and improve performance , easy to prepare and obtainable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
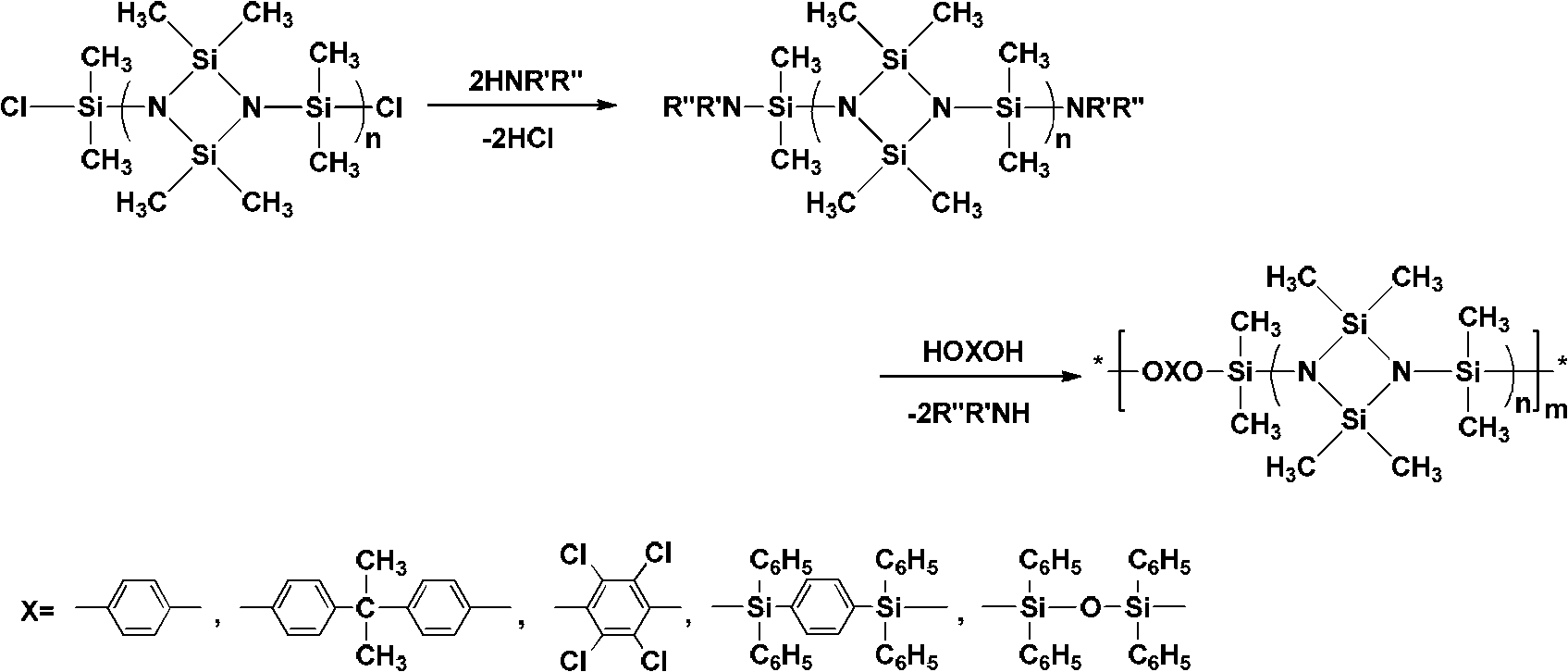
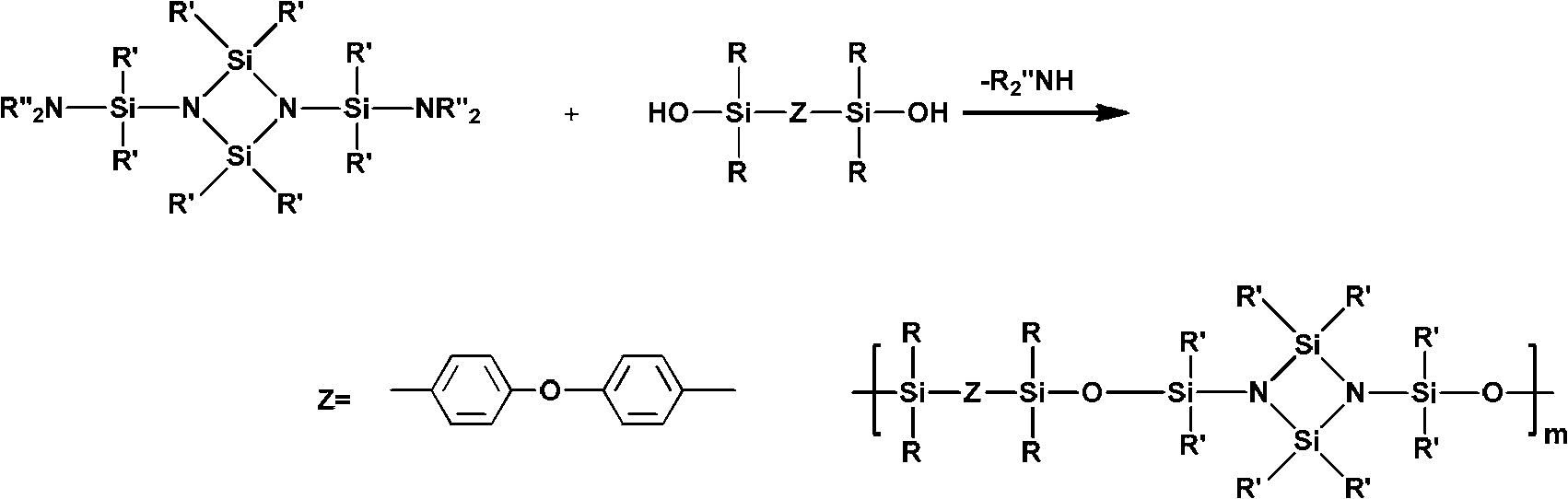
[0055] Raw materials used for synthesis: the molecular formula is as shown in the above-mentioned formulas II-IV, wherein, in the formula II, R 1 = R 2 =CH 3 ; In Formula IV, In Formula III, n=3.
[0056] Under a nitrogen atmosphere, 5.42g (0.01mol) of 1,3-bis(methylphenylhydroxysilyl)-2,4-dimethyl-2,4-diphenylcyclodisilazane (molecular formula shown in formula II) was dissolved in 20mL toluene, and 8.48g (0.02mol) α, ω-diethylaminosiloxane (molecular formula shown in formula III, n=3) (Aldrich Chemical Co.) and 2.93g (0.01mol) 1,3-bis(hydroxydimethylsilyl)-m-carborane (molecular formula as shown in formula IV), fully mixed evenly. Slowly raise the temperature to 110°C, keep mechanical stirring, blow out the generated diethylamine continuously with nitrogen until no more diethylamine is generated. The reaction was carried out for a total of 12 hours. After the reaction was completed, toluene was added to dissolve the polymer, and anhydrous methanol was used to precipitat...
Embodiment 2
[0061] Raw materials used for synthesis: the molecular formula is as shown in the above-mentioned formulas II-IV, wherein, in the formula II, R 1 = R 2 =C 6 h 5 ; In Formula IV, In Formula III, n=3.
[0062] Under a nitrogen atmosphere, 6.66g of 1,3-bis(diphenylhydroxysilyl)-2,4-dimethyl-2,4-diphenylcyclodisilazane (molecular formula shown in formula II) (0.01mol) dissolved in 20mL xylene, continue to add 8.48g (0.02mol) α, ω-diethylaminosiloxane (molecular formula shown in formula III, n=3) (Aldrich Chemical Co.) and 2.93g (0.01mol) 1,3-bis(hydroxydimethylsilyl)-m-carborane (molecular formula as shown in formula IV), fully mixed evenly. Slowly raise the temperature to 140°C, keep mechanical stirring, blow out the generated diethylamine continuously with nitrogen until no more diethylamine is generated. The reaction was carried out for a total of 12 hours. After the reaction was completed, n-hexane was added to dissolve the polymer, and precipitated with anhydrous metha...
Embodiment 3
[0067] Synthetic raw materials used: as shown in the above-mentioned formula II~IV, wherein, in formula II, R 1 = R 2 =CH 3 ; In Formula IV, In Formula III, n=3.
[0068] Under nitrogen atmosphere, 5.42g 1,3-bis(methylphenylhydroxysilyl)-2,4-dimethyl-2,4-diphenylcyclodisilazane (molecular formula shown in formula II ) (0.01mol) and 2.26g (0.01mol) 1,3-bis(hydroxydimethylsilyl)benzene (molecular formula shown in formula IV) (Shanghai Merrill Chemical Technology Co., Ltd.) was dissolved in 40mL petroleum ether (bp .90~120°C) and toluene (volume ratio=1:1), continue to add 8.48g α, ω-diethylaminosiloxane (molecular formula shown in formula III, n=3) (Aldrich Chemical Co.) (0.02mol), mix well. Slowly raise the temperature to 105°C, keep mechanical stirring, blow out the generated diethylamine continuously with nitrogen until no more diethylamine is generated. The reaction was carried out for a total of 12 hours. After the reaction was completed, xylene was added to dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com