Anti-CD4 protein monoclonal antibody and its active fragment and application
A monoclonal antibody and protein technology, applied in the fields of prevention, immunotherapy and diagnosis, can solve problems such as interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] Example 1. Can inhibit HIV infection CD4 + Cellular Anti-CD4 Monoclonal Antibody Preparation
[0191] Mice: 6-week-old female BALB / c mice were provided by the Experimental Animal Center of School of Life Sciences, Xiamen University.
[0192] Preparation of hybridomas: We use standard in vivo immunization methods and PEG fusion methods to obtain monoclonal antibodies. For detailed methods, see Ed Harlow et al., Antibodies A Laboratory Manual, Cold Spring Harbor Laboratory 1988. The brief process is as follows:
[0193] Mice immunization: mix and emulsify the recombinant sCD4 antigen solution dissolved in PBS and complete Freund's adjuvant (CFA) in equal volumes, inject at multiple points through the muscles of the limbs, and inject 5 μg of the purified recombinant sCD4 antigen prepared in Example 2 per mouse (total volume 50 μL). 15 days and 29 days after the first immunization, booster immunization was carried out with the same dose of recombinant antigen solution plu...
Embodiment 2
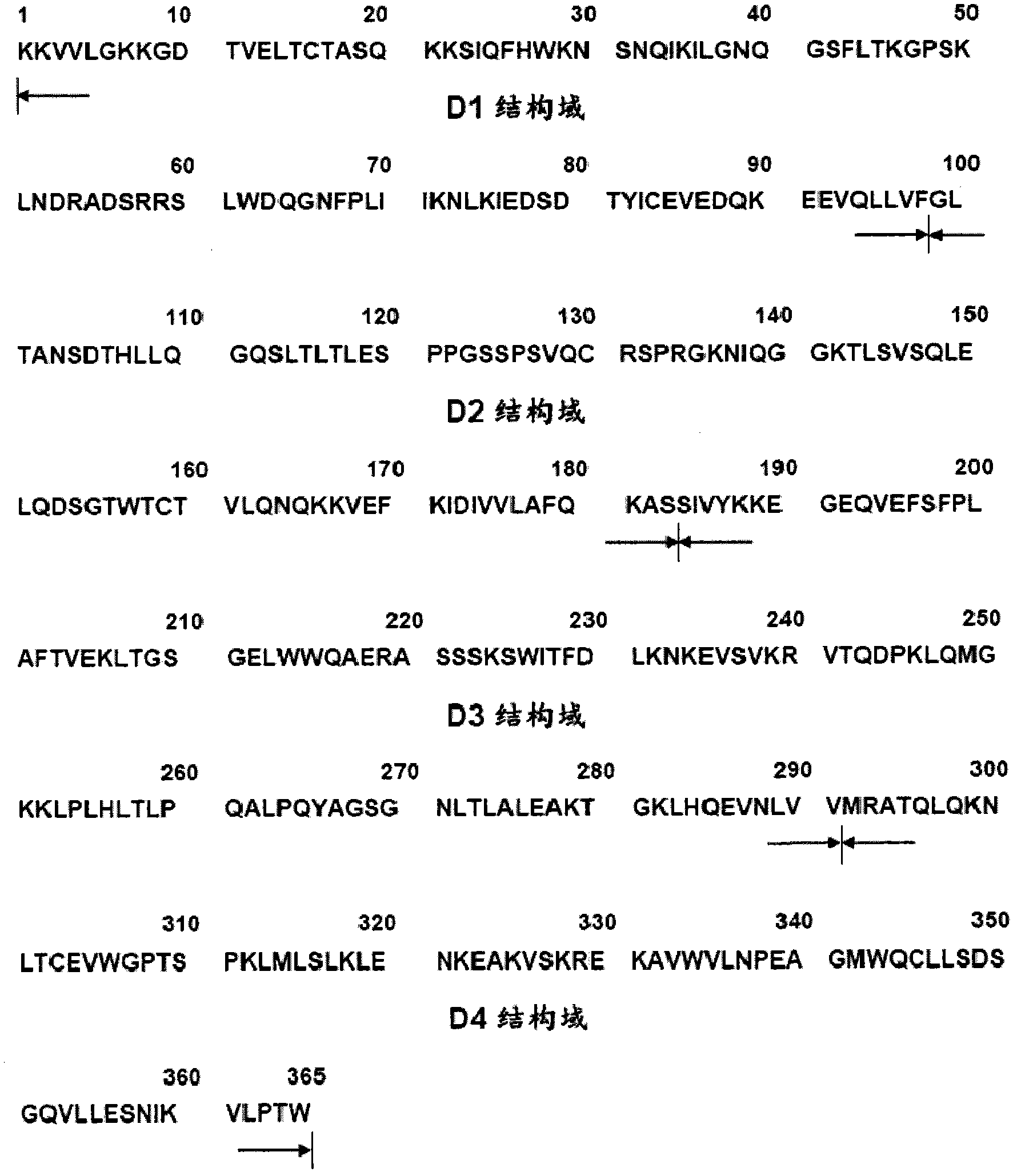
[0200] Example 2. Preparation of Recombinant Human CD4 Fragment Polypeptide Used as Antigen
[0201] Preparation of human CD4 ectoregion fragments used as template: human CD4 cDNA in TZM-bl cell line (Cat. -CGG CATATG AAG AAA GTG GTG CTG GGC-3′) is the forward primer, CD4(d4)R (5′-GCGAATTCTTACCATGTGGGCAGAACCTT-3′) is the reverse primer, and the PCR thermal cycler (Biometra T3) is carried out according to the following conditions PCR reaction: 94°C for 10 minutes; followed by 25 cycles of 94°C for 30 seconds, 56°C for 30 seconds, 72°C for 1 minute, and finally 72°C for 10 minutes. A specific 1.1 kb template DNA fragment was obtained and used as a template for preparing each truncated recombinant human CD4 polypeptide. The PCR product obtained above was ligated with a commercially available pMD 18-T vector (manufactured by TaKaRa Company), and identified by Nde I / EcoR I digestion to obtain a positive clone inserted into the sCD4 gene. Using M13(+) / (-) primers, the conserved se...
Embodiment 3
[0215] Example 3. Monoclonal antibody blocking HIV infection TZM-bl cell activity verification
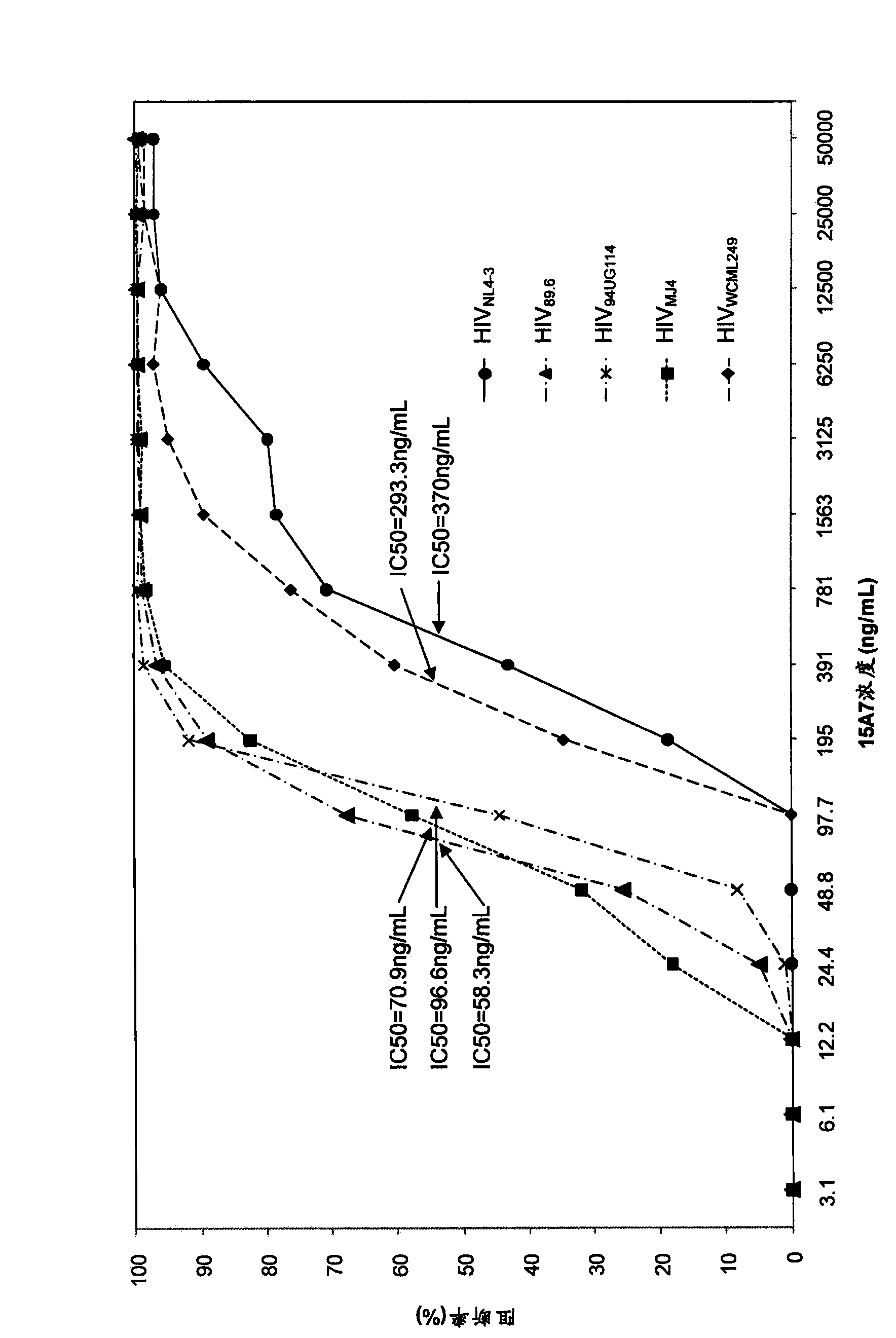
[0216] Select 5 strains to belong to respectively B, C, D, D / C subtype HIV full-gene experimental strain virus (HIV NL4-3 , HIV 89.6 , HIV 94UG114 , HIV MJ4 , HIV WCML249 ), through the cell neutralization experiment to determine the monoclonal antibody 15A7, 14G7 to the above virus infection CD4 + Cellular blocking / neutralizing effect. TZM-bl cells according to 1.5 × 10 4 The concentration of cells / well was inoculated in 96-well cell culture plate and used for detection after 12 hours. as per image 3 Dilute monoclonal antibodies 15A7 and 14G7 at the dilution concentrations listed in the coordinates, add 100 μL of antibody diluent and 50 μL of each HIV virus suspension (diluted to 100 TCID50) in each well of a 96-well U-bottom plate, and incubate at 37°C for 1 hour. 150 μL of the mixture was added to the cultured TZM-bl cells on a 96-well cell culture plate, and cultured a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com