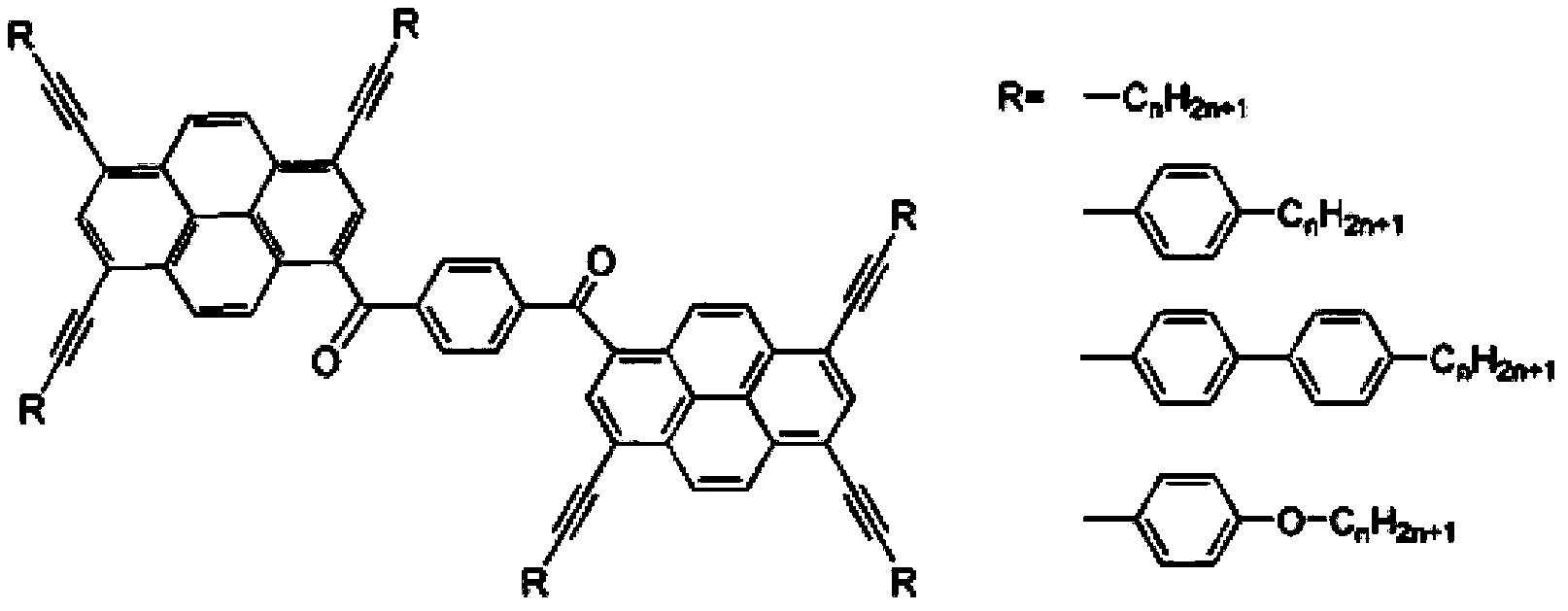
Pyrene large conjugated system disciform liquid crystal compound and preparation method thereof
A technology of conjugated system and discotic liquid crystal, which is applied in the field of discotic liquid crystal compound and its preparation, can solve rare problems and achieve the effect of wide application prospect and good carrier transport efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
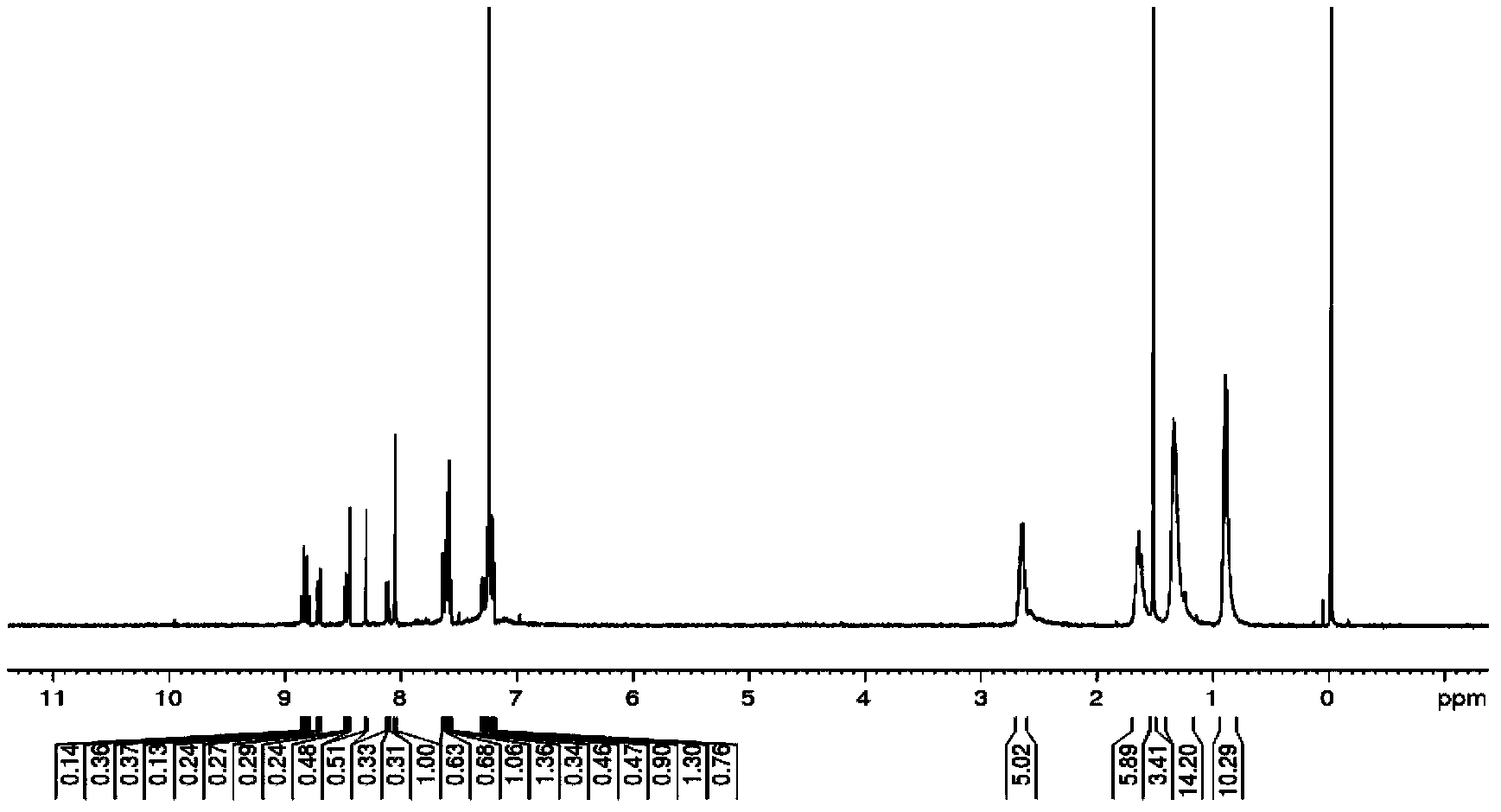
[0019] Take the R structure as: -C 5 h 11 The synthetic method of this compound is introduced as an example
[0020] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide, and place it in a 50mL single-necked bottle, add 1.5mmol acid chloride (3.26g), cool to 0 degrees in an ice bath, 0.02mol Aluminum trioxide (2.64g), heated to reflux for 16 hours, cooled the product and poured it into 100mL of ice water and stirred for 2 hours, extracted with dichloromethane, dried and filtered the organic phase through anhydrous magnesium sulfate, and recrystallized to obtain the product (a) 2.76g, yellow needle-like crystals, yield 72%.
[0021] 2: intermediate product bromination reaction: 5mmol product (a) (1.92g) gained in step 1 is dissolved in 50mL nitrobenzene, is placed in the 100mL single-necked bottle, and 20mmol liquid bromine (1.2mL) is gradually added dropwise at room temperature, After the dropwise addition, the reaction was st...
Embodiment 2
[0024] Take the R structure as: The synthetic method of this compound is introduced as an example
[0025] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide, and put it in a 50mL single-necked bottle, add 1.5mmol acid chloride (3.26g), cool to 0 degrees in an ice bath, add 0.02 mol of aluminum trioxide (2.64g), heated and refluxed for 12 hours, cooled the product and poured it into 100mL of ice water and stirred for 2 hours, separated the liquids with dichloromethane, dried and filtered the organic phase through anhydrous magnesium sulfate, and then recrystallized to obtain the intermediate product (a) 2.76g, yellow needle-like crystals, yield 72%.
[0026] 2: Intermediate product bromination reaction: 5 mmol product (a) (1.92 g) obtained in step 1 was dissolved in 50 mL nitrobenzene, placed in a 100 mL single-necked bottle, and 20 mmol liquid bromine (12 mL) was gradually added dropwise at room temperature. After the addit...
Embodiment 3
[0028] Example 3: The synthetic method of this compound is introduced as an example
[0029] 1: Friedel-Crafts acylation reaction of pyrene: Dissolve 10mmol pyrene (2g) in 30mL carbon disulfide and put it in a 50mL single-necked bottle, add 1.5mmol acid chloride (3.26g), cool to 0 degrees in an ice bath, add 0.02 mol of aluminum trioxide (2.64g); heated to reflux for 12 hours; the product was cooled and poured into 100mL of ice water and stirred for 2 hours, extracted with dichloromethane, and the organic phase was recrystallized after drying and filtering over anhydrous magnesium sulfate to obtain the product (a ) 2.76g, yellow needle-like crystals, yield 72%.
[0030] 2: intermediate product bromination reaction: 5mmol product (a) (1.92g) gained in step 1 is dissolved in 50mL nitrobenzene, is placed in the 100mL single-necked bottle, and 20mmol liquid bromine (1.2mL) is gradually added dropwise at room temperature, After the dropwise addition, the temperature was raised t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com