Oral slow release preparation, entrapment material and preparation method
A technology of sustained-release preparations and encapsulation materials, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Effect of long-term action and high biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
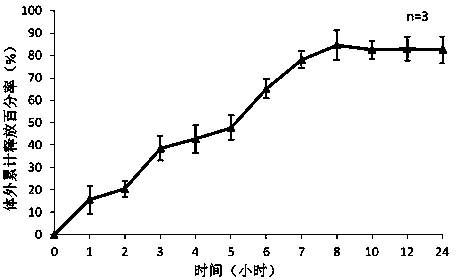
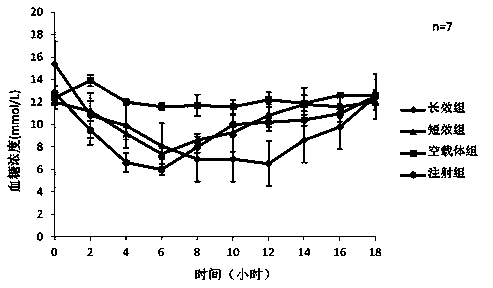
[0042] Weigh 5mg of exenatide, sodium alginate:sodium hyaluronate=2:3, dissolve the mixture of exenatide, sodium alginate and sodium hyaluronate in three distilled water, the concentration is 25mg / ml, stir and dissolve To obtain the water phase, add 2% Span-80 to 20ml of liquid paraffin to obtain the oil phase, add the water phase to the oil phase drop by drop, and magnetically stir at 500rpm / min to disperse the water phase evenly in the oil phase; add to the oil phase 2ml of 25% glutaraldehyde solution and 10ml of 1M dilute hydrochloric acid were reacted at 20°C; after the reaction was completed, 20ml of ethanol was added and stirred at room temperature for 15 minutes. The preparation is complete after freeze-drying. Weigh 100 mg of drug-loaded exenatide microspheres and 100 mg of blank microspheres prepared under the same conditions, and place each in a 20ml system to measure the encapsulation efficiency, and place them in a hydrochloric acid solution with pH 1.2 for the fir...
Embodiment 2
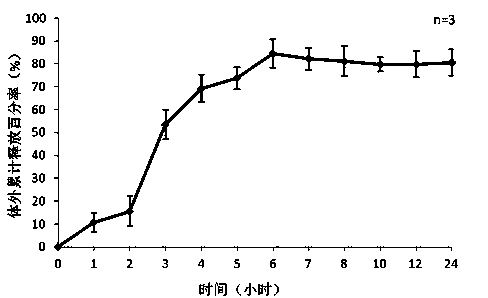
[0044] Weigh 10mg of exenatide, sodium alginate: sodium hyaluronate=1:1, dissolve the mixture of exenatide, sodium alginate and sodium hyaluronate in three distilled water, the concentration is 20mg / ml, stir and dissolve To obtain the water phase, add 1% Span-80 to 50ml of liquid paraffin to obtain the oil phase, add the water phase to the oil phase drop by drop, and stir the water phase at 700rpm / min to uniformly disperse the water phase in the oil phase; add 20ml 25% glutaraldehyde solution and 10ml 1M dilute hydrochloric acid, react at 50°C; after the reaction, add 20ml absolute ethanol, stir at room temperature for 15 minutes, discard the upper layer after standing for separation, and wash the lower layer with ethanol after suction filtration times, the preparation is complete after freeze-drying. The encapsulation rate of exenatide microspheres determined by BCA method was 78%, and the particle size was <200 μm.
Embodiment 3
[0046] Weigh exenatide 10mg, sodium alginate: sodium hyaluronate=1:3, dissolve the mixture of exenatide, sodium alginate and sodium hyaluronate in three distilled water, the concentration is 40mg / ml, stir and dissolve To obtain the water phase, add 1% Span-80 to 20ml of liquid paraffin to obtain the oil phase, add the water phase to the oil phase drop by drop, and magnetically stir at 500rpm / min to disperse the water phase evenly in the oil phase; add to the oil phase 5ml 25% glutaraldehyde solution and 5ml 1M dilute hydrochloric acid, react at 25°C, add 20ml ethanol after the reaction, stir at room temperature for 15 minutes, discard the upper layer after static separation, wash the lower layer with ethanol several times after suction filtration, freeze After drying, the preparation is complete. The encapsulation rate of exenatide microspheres determined by BCA method was 86%, and the particle size was <220 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com