Method for removing fluorions in drinking water by strengthened magnesium precipitations
A fluoride ion, drinking water technology, applied in the field of water treatment, can solve the problems of complicated operation and high water treatment cost, achieve good removal effect, high fluoride removal efficiency, and promote the effect of magnesium ion precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
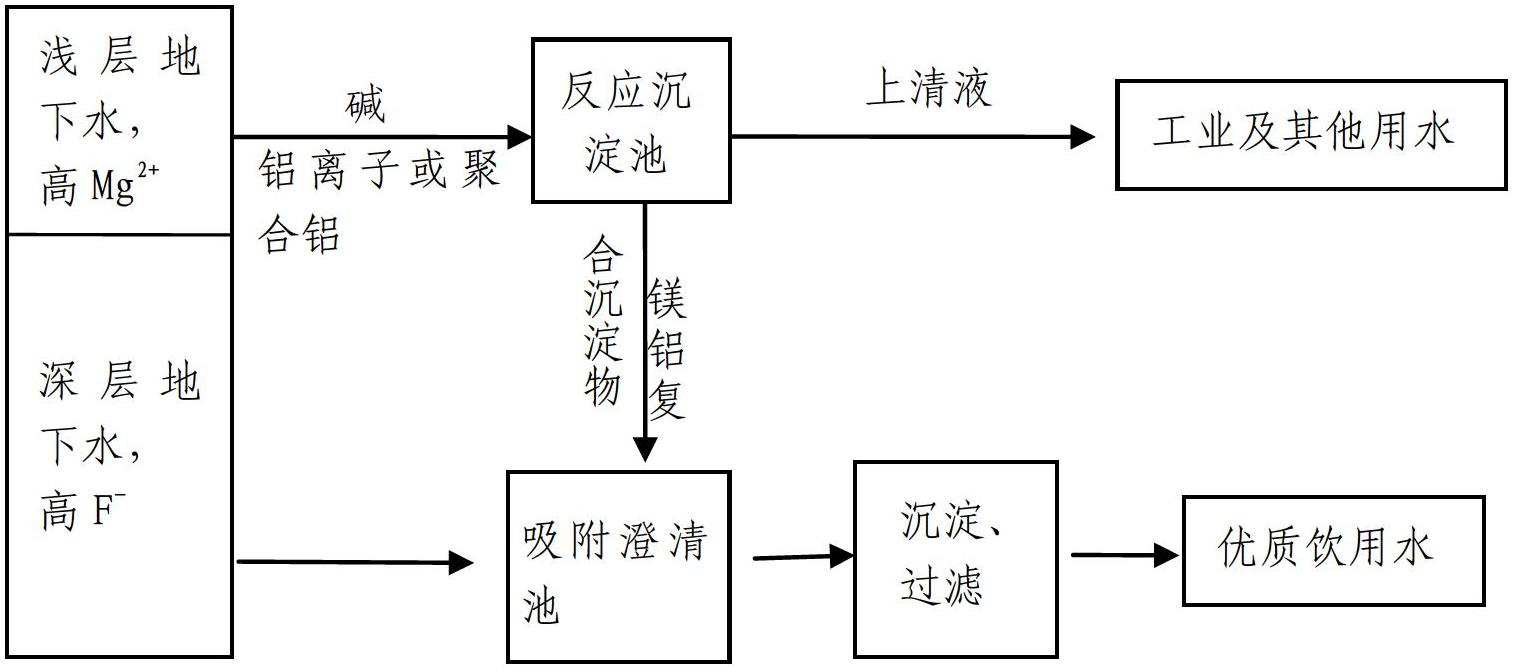
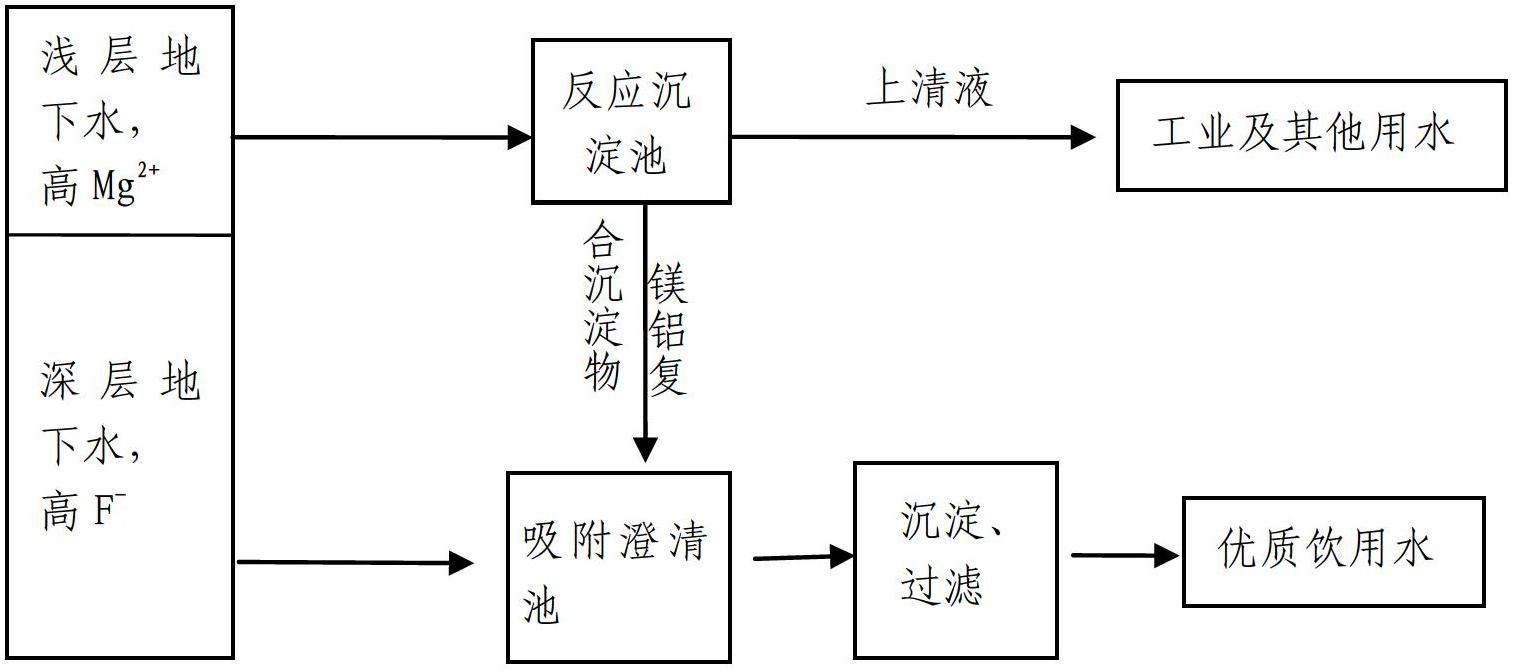
[0025] Embodiment 1. Comprehensive purification method of high hardness shallow water and high fluorine water
[0026] (1) Preparation of a solution containing Al: dissolving solid polyaluminum chloride PACl to prepare an Al solution with an aluminum content of 0.2 mol / L.
[0027] (2) Prepare 1.0mol / L NaOH solution.
[0028] (3) Extract shallow high-hardness groundwater with a magnesium ion concentration of 50mg / L, enter the reaction tank, and put the above-mentioned NaOH and aluminum ion-containing solution into it, so that the pH of the solution is 10.5, and the Al concentration is 0.10mmol / L , rapid stirring, the hydraulic retention time of the reaction tank is controlled at 20 minutes, then it is introduced into the sedimentation tank, and the sedimentation is static for 30 minutes. The effluent of the sedimentation tank is used as industrial and other water. It is discharged from the bottom of the sedimentation tank into the adsorption clarification tank, where it is mix...
Embodiment 2
[0029] Embodiment 2, strengthening co-precipitation purification method:
[0030] (1) Prepare a solution containing Al: dilute the aluminum chloride solution to prepare a 0.2mol / L Al solution.
[0031] (2) Preparation of MgO powder;
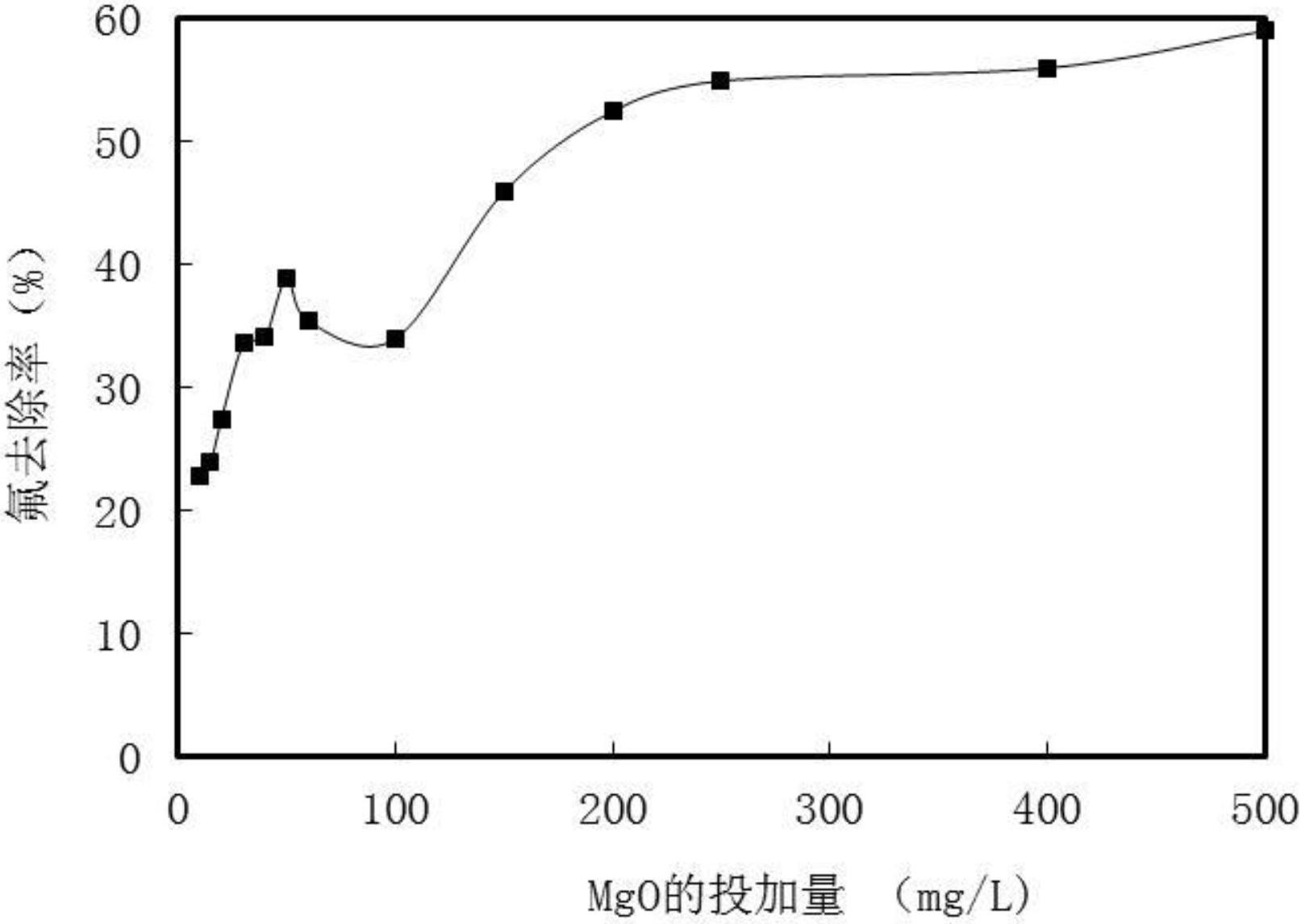
[0032] (3) Add MgO powder to the water containing 2.5mg / L of fluoride ion to make the pH of the solution 8.5-11.2. The pH of the solution increases with the increase of the dosage of MgO. At the same time, add 0.2mol / L L Al solution until the Al concentration is 0.06mmol / L, stir rapidly for 10 minutes, form a magnesium-aluminum composite co-precipitation to remove fluoride ions during the strengthening process, precipitate for 1 hour, remove the sediment, and the upper clarified water is purified and treated high-quality drinking water . The relationship curve between fluoride ion removal rate and MgO dosage, such as figure 2 As shown, when the dosage of MgO powder is 200mg / L, the pH of the solution is 10.5, the fluoride ion concentration aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com