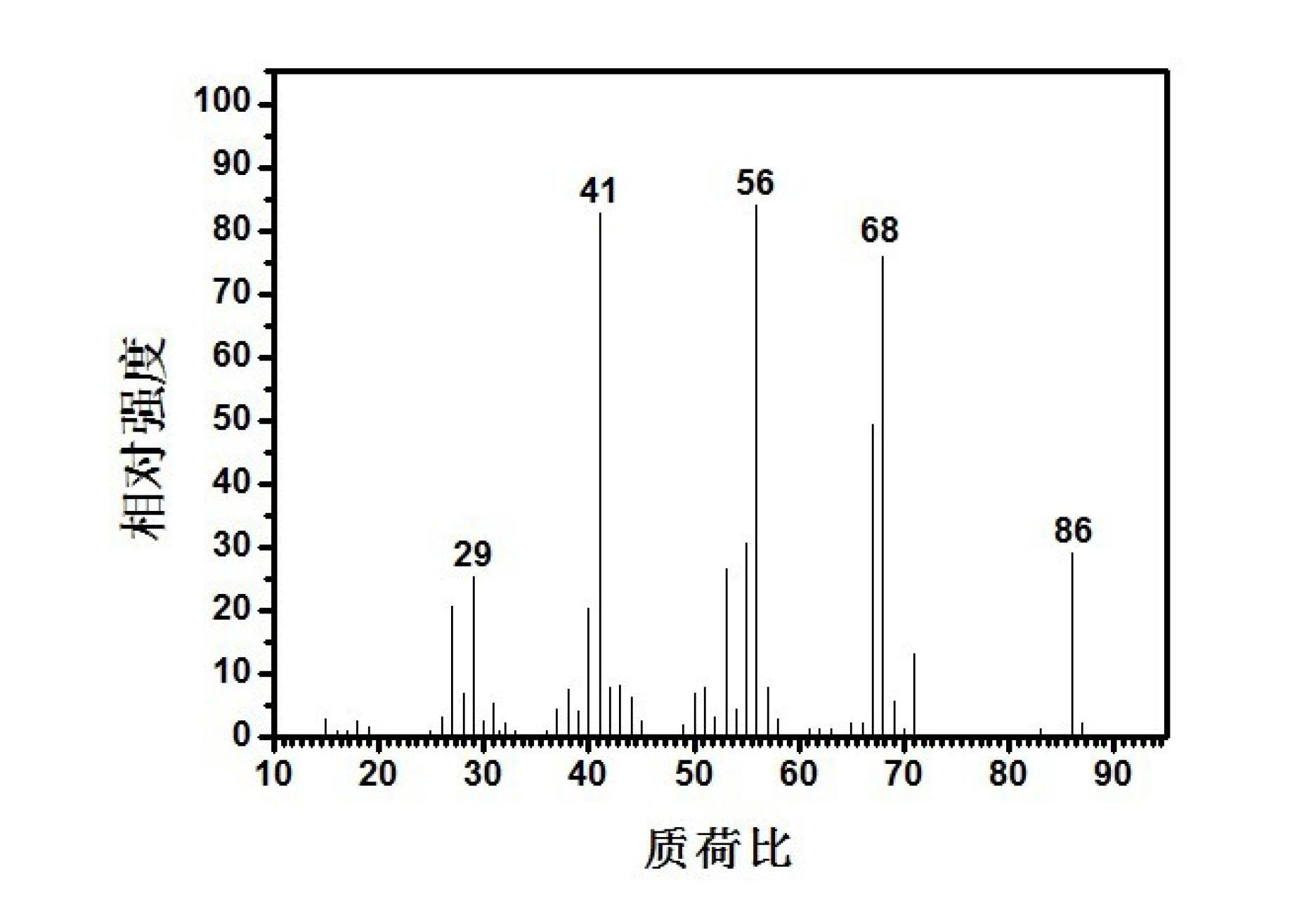
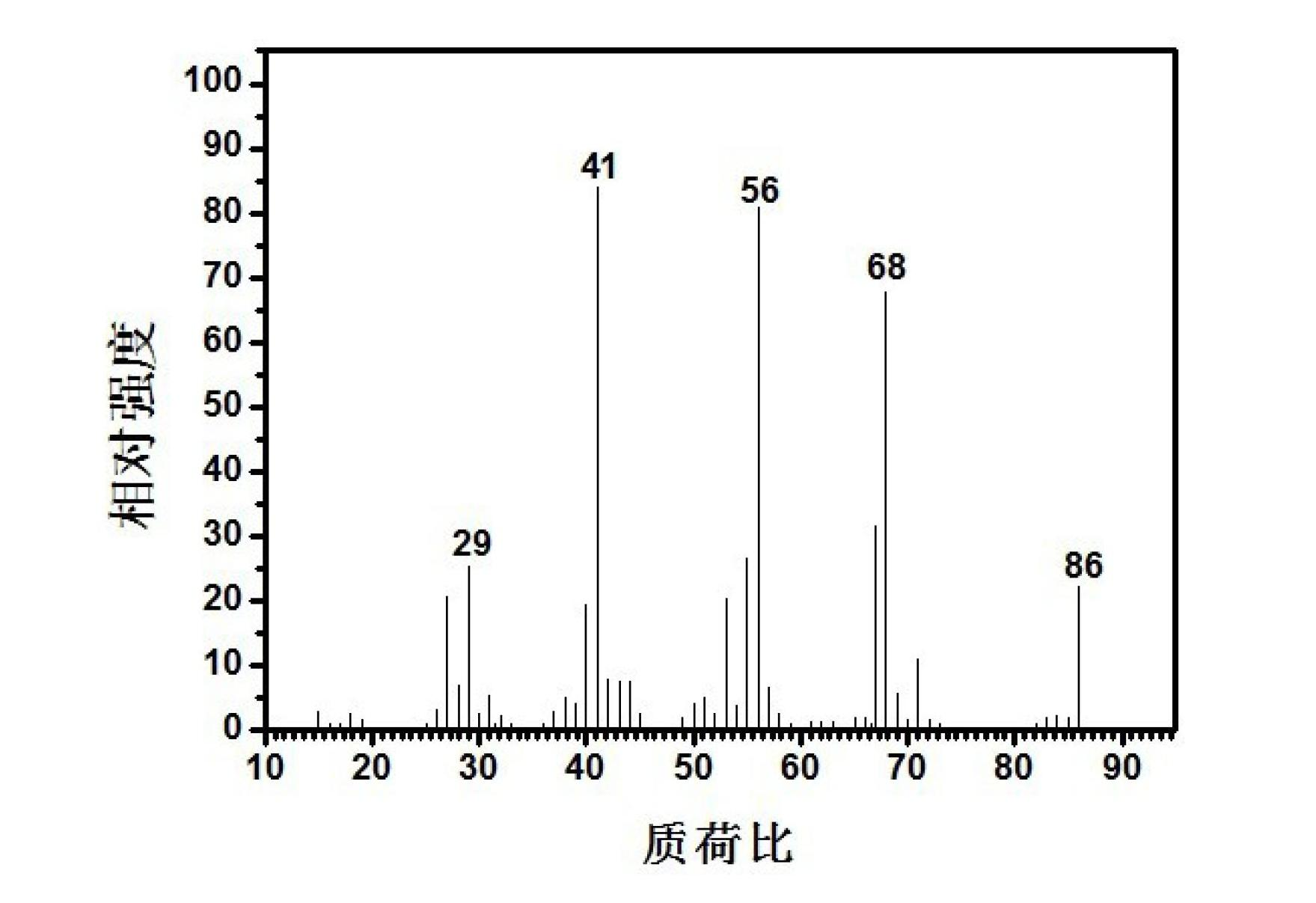
Method for preparing 3-methyl-3-butylene-1-alcohol
A technology of butene and methyl, which is applied in the field of catalytic synthesis, can solve the problems of high reaction temperature, high reaction pressure, acid catalyst corrosion, etc., and achieve the effect of solving the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The above-prepared 3gSnCl 2 The solid-supported catalyst is placed in the middle of a stainless steel fixed-bed reaction tube with an inner diameter of 8 mm and a length of 300 mm, and the upper and lower parts of the catalyst are filled with 40-60 mesh quartz sand; 2 The air in the fixed-bed reactor was purged clean, and the temperature was raised to 120°C to be constant. Put 100g of polyoxymethylene solid in a polyoxymethylene depolymerization spherical glass container, and raise the temperature to 150°C and keep it constant. Cut off N 2 , the flow rate is 30ml / min C 4 The mixed gas (the mass fraction of isobutylene is 10%) is passed into the spherical glass container of polyoxymethylene solid depolymerization, carrying the gaseous formaldehyde generated by the depolymerization of polyoxymethylene, and enters the fixed bed reactor equipped with catalyst. The speed is 10.4h -1 , to carry out the catalytic reaction of synthesizing 3-methyl-3-buten-1-ol. At this tim...
Embodiment 2
[0044] The above-prepared 3gSnCl 2 The solid-supported catalyst is placed in the middle of a stainless steel fixed-bed reaction tube with an inner diameter of 8 mm and a length of 300 mm, and the upper and lower parts of the catalyst are filled with 40-60 mesh quartz sand; 2 The air in the fixed-bed reactor was purged clean, and the temperature was raised to 120°C to be constant. Put 100g of polyoxymethylene solid in a spherical glass container for polyoxymethylene depolymerization, and raise the temperature to 200°C and keep it constant. Cut off N 2 , the flow rate is 30ml / min C 4 The mixed gas (the mass fraction of isobutylene is 10%) is passed into the spherical glass container of polyoxymethylene solid depolymerization, carrying the gaseous formaldehyde generated by the depolymerization of polyoxymethylene, and enters the fixed bed reactor equipped with catalyst. The speed is 16.6h -1 , to carry out the catalytic reaction of synthesizing 3-methyl-3-buten-1-ol. At this...
Embodiment 3
[0046] The above-prepared 3gSnCl 2 The solid-supported catalyst is placed in the middle of a stainless steel fixed-bed reaction tube with an inner diameter of 8 mm and a length of 300 mm, and the upper and lower parts of the catalyst are filled with 40-60 mesh quartz sand; 2 The air in the fixed-bed reactor was purged, and the temperature was raised to 180°C to be constant. Put 100g of polyoxymethylene solid in a polyoxymethylene depolymerization spherical glass container, and raise the temperature to 150°C and keep it constant. Cut off N 2 , the flow rate is 30ml / min C 4 The mixed gas (wherein the mass fraction of isobutylene is 10%) passes into the spherical glass container of polyoxymethylene solid depolymerization, carrying the gaseous formaldehyde generated by the depolymerization of polyoxymethylene, and enters the fixed bed reactor equipped with catalyst. The mass space velocity of formaldehyde is 10.4h -1 , to carry out the catalytic reaction of synthesizing 3-meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com