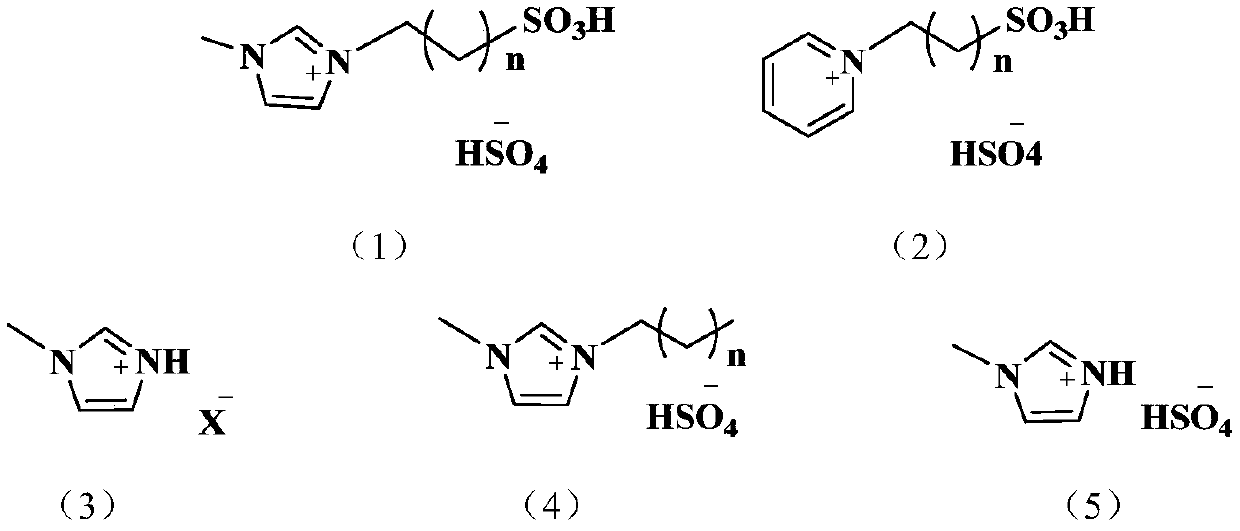
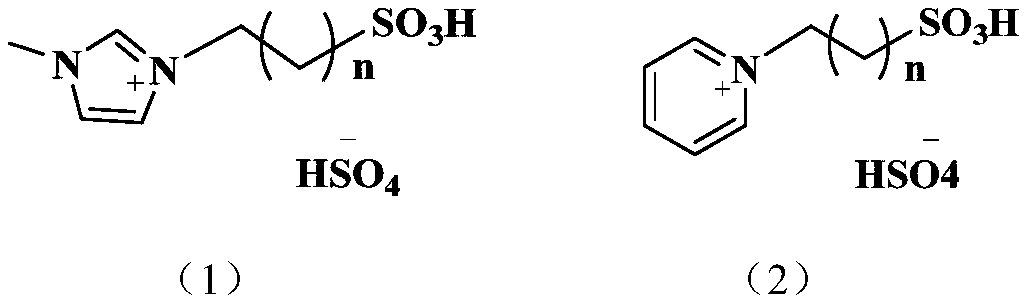
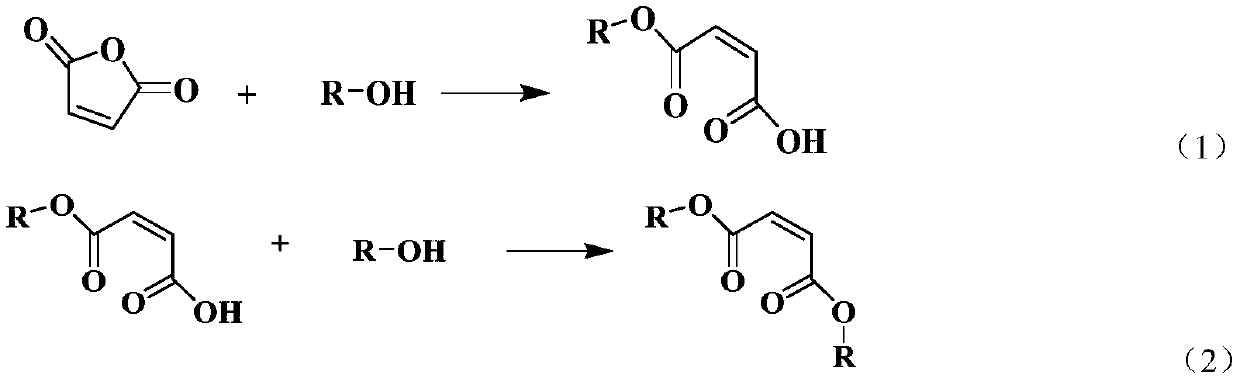
Method for preparing maleate by catalyzing maleic anhydride with ionic liquid
A technology of maleic anhydride and ionic liquid, which is applied in the field of ionic liquid catalyzing maleic anhydride to prepare maleic acid ester, can solve the problems of difficult complete regeneration, equipment corrosion, and many side reactions, and achieves the effect of solving equipment corrosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of Dimethyl Maleate by the Reaction of Maleic Anhydride and Methanol Catalyzed by 1-Propylsulfonic Acid-3-Methylimidazolium Bisulfate Ionic Liquid
[0027] Preparation of 1-propylsulfonic acid-3-methylimidazolium bisulfate ionic liquid:
[0028] Add 4.106g (0.05moL) of methylimidazole and ethanol (50mL) into a 150mL three-necked flask, preheat it in an oil bath at 70°C, and add 1,3-propylsulfonic acid dropwise under magnetic stirring Add 6.107 g (0.05 mol) of the ester into the flask. After the dropwise addition, continue stirring for 8 h to obtain the intermediate product, 1-propylsulfonic acid-3-methylimidazole with cationic sulfonic acid function. After the reaction, the intermediate product was washed with ether for 3 to 5 times to remove unreacted raw materials, and then put into a vacuum drying oven and dried at 80° C. for 12 hours.
[0029] Add the intermediate product into a 150mL round-bottomed flask, dissolve it with an appropriate amount of deion...
Embodiment 2
[0032] Preparation of Dimethyl Maleate by Reaction of Maleic Anhydride and Methanol Catalyzed by N-Butyl Sulfonate Pyridinium Bisulfate Ionic Liquid
[0033] Preparation of N-butylsulfonic acid pyridinium bisulfate ionic liquid:
[0034] Add 3.933g (0.05mol) of pyridine and ethanol (50mL) into a 150mL three-necked flask, preheat it in an oil bath at 70°C, and add 6.8085g (0.05mol) of butyl sultone dropwise under magnetic stirring. ) into the flask, after the dropwise addition, continue to stir for 8h to obtain the intermediate product, namely the N-butylpyridinesulfonic acid function of the cationic sulfonic acid. After the reaction, the intermediate product was washed with ether for 3 to 5 times to remove unreacted raw materials, and then put into a vacuum drying oven and dried at 80° C. for 12 hours.
[0035] Add the intermediate product into a 150mL round-bottomed flask, dissolve it with an appropriate amount of deionized water, preheat it at 70°C, then add an equimolar am...
Embodiment 3
[0038] Preparation of Diethyl Maleate by Reaction of Maleic Anhydride and Ethanol Catalyzed by 1-Propylsulfonic Acid-3-Methylimidazolium Bisulfate Ionic Liquid
[0039] Preparation of 1-propylsulfonic acid-3-methylimidazolium bisulfate ionic liquid: the process is the same as in Example 1.
[0040] Esterification of maleic anhydride: Weigh maleic anhydride 0.9806g (0.01mol), ethanol 2.7642g (0.06mol), 1-propylsulfonic acid-3-methylimidazolium bisulfate ionic liquid 0.0605g (0.2mmol) ) in a glass microwave tube, set the reaction temperature to 120° C., reacted for 1 hour, the conversion rate of maleic anhydride reached 97.87%, and the yield of diethyl maleate reached 88.39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com