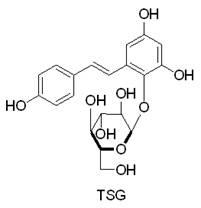
Separation and purification process of tetrahydroxy stilbeneglycoside
A tetrahydroxy stilbene, separation and purification technology, applied in organic chemistry, chemical instruments and methods, preparation of sugar derivatives, etc., can solve the problems of large sample loss, low recovery rate, unstable separation and purification, etc., to simplify separation Purification steps, overall process conditions are mild, soluble and stable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
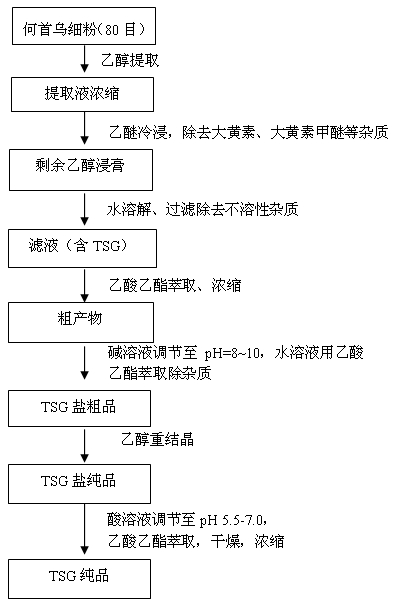
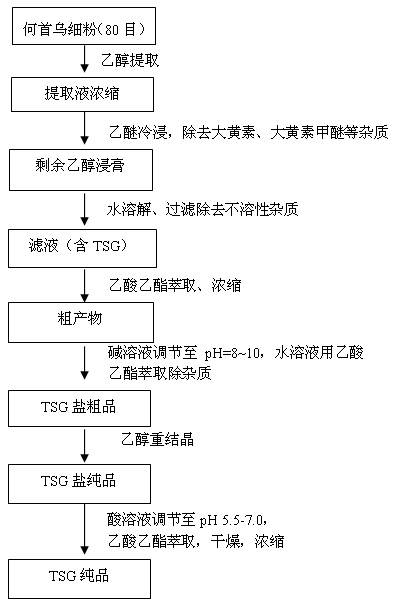
[0035] Weigh 100 g (80 mesh) of Radix Polygoni Multiflori powder and put it in a beaker, add 200 mL of industrial ethanol, ultrasonically extract for 10 minutes, let stand, separate the supernatant, and spin off the solvent to obtain a brown extract. In the brown extract, add diethyl ether to soak, and filter to remove the ether layer after 0.5 h. The extracted extract after cold soaking in ether is fully dissolved by adding water, and the insoluble impurities are removed by filtration. The filtrate was extracted with ethyl acetate until the ester layer had no absorption point. The ester layer was collected and the solvent was removed to obtain a brown crude product. The crude product was adjusted to pH=9 with 1mol / L sodium hydroxide, and extracted with ethyl acetate until the ester layer had no absorption point. Leave the water layer. Cyclohexane was added to the water layer, and ethanol and water formed an azeotrope (the volume ratio of the three was 70%:7%:23%, and the b...
Embodiment 2
[0037]Weigh 500 g (80 mesh) of Radix Polygoni Multiflori powder and put it in a beaker, add industrial ethanol and ultrasonically extract for 10 minutes, let it stand, separate the supernatant, and spin off the solvent to obtain a brown extract. In the brown extract, add diethyl ether to soak, and filter to remove the ether layer after 0.5 h. Add water to fully dissolve the extracted extract after ether cold soaking, and filter to remove insoluble impurities. The filtrate was extracted with ethyl acetate until the ester layer had no absorption point. The ester layer was collected and the solvent was removed to obtain a brown crude product. The crude product was adjusted to pH=8.5 with 1mol / L sodium bicarbonate, and extracted with ethyl acetate until the ester layer had no absorption point. Leave the water layer. Cyclohexane was added to the water layer, ethanol and water formed an azeotrope, and the solvent was spun off to obtain crude TSG salt. The crude product was recry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com