Erythromycin ethylsuccinate crystalline hydrate and preparation method and application thereof
A technology of erythromycin and compounds, applied in the field of medicine, to achieve good storage stability at room temperature, easy storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
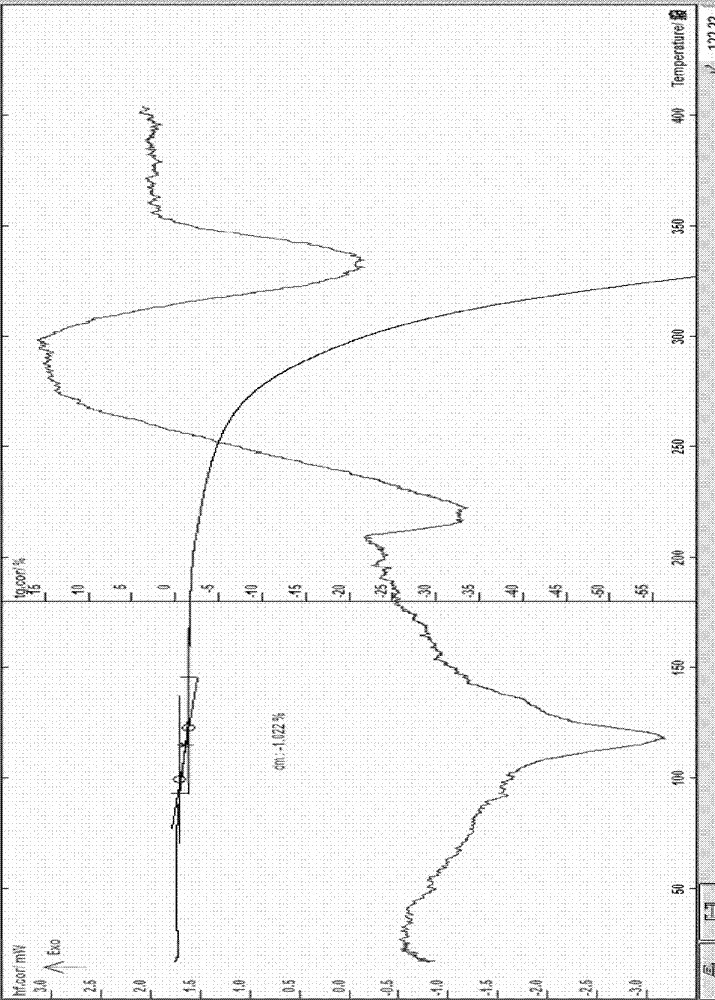
[0058] Example 1 Preparation of erythromycin ethylsuccinate 0.5 hydrate: in a three-necked flask, add 35ml of tetrahydrofuran, 10ml of acetone, 8g of erythromycin, 15ml of 47% potassium carbonate, 10ml of water and stir, cool to below 15°C, add Succinic acid monoethyl chloride 4.8g, monitor by TLC until the reaction is complete, slowly add 5M sodium phosphate aqueous solution, stir, control the pH value at about 7.0-8.5, settle and separate the organic phase, slowly add about 60ml into the organic phase under stirring of water, cooled to -15~0°C, after the solid precipitation is complete, filter, the obtained solid is recrystallized with ethanol and water, cooled to about -10°C, after the solid precipitation is complete, suction filtration, the obtained solid is at about 50°C After drying for 8 hours, 4.2 g of off-white solid was obtained; melting point: 109.2~111.7°C (uncorrected), this sample was tested according to the identification items (1) and (2) of erythromycin ethylsu...
Embodiment 2
[0059] Example 2 Preparation of erythromycin ethylsuccinate 0.5 hydrate: In a three-necked flask, add 40ml of acetone, 50ml of tetrahydrofuran, 20g of erythromycin thiocyanate, stir at 30-45°C, add 40ml of 12g potassium carbonate aqueous solution, and stir to react For 1 hour, add 4M sodium dihydrogen phosphate aqueous solution dropwise, stir, control the pH value at about 7.0-8.5, settle and separate the organic phase, add 40ml of 47% potassium carbonate and 10ml of water to the organic phase, stir, cool to below 15°C, Add 10 grams of monoethyl succinic acid chloride, monitor with TLC until the reaction is complete, add dropwise 5M sodium citrate aqueous solution, stir, control the pH value at about 7.0-8.5, settle and separate the organic phase, slowly add to the organic phase while stirring About 100ml of water, cooled to -15 ~ 0 ℃, after the solid precipitation is complete, suction filtration, the obtained solid is recrystallized with isopropanol and water, suction filtrati...
Embodiment 3
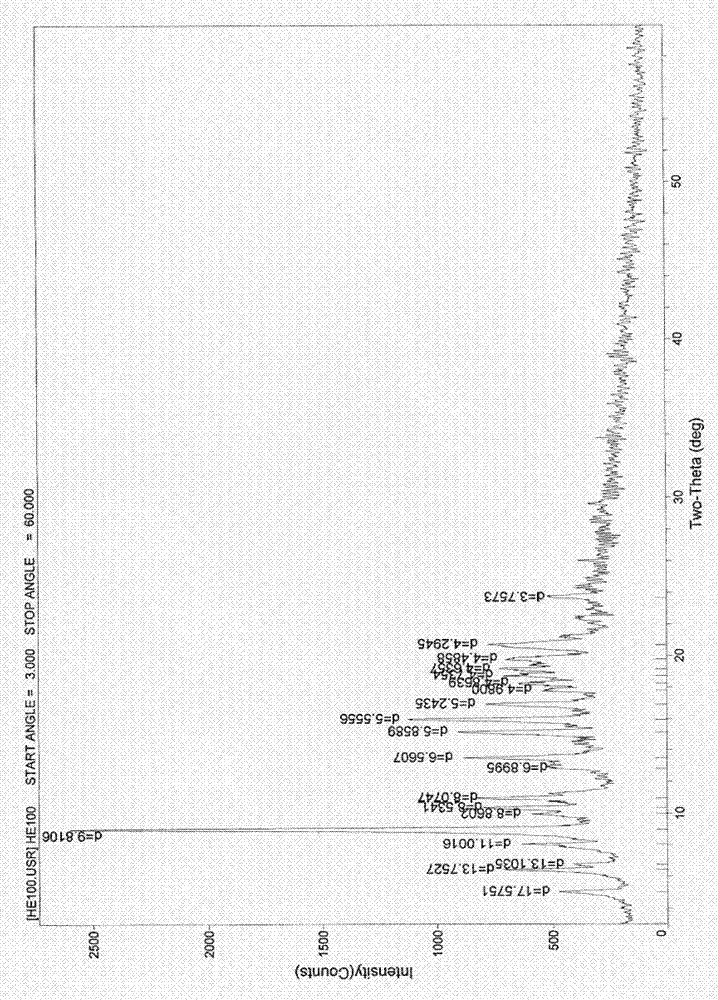
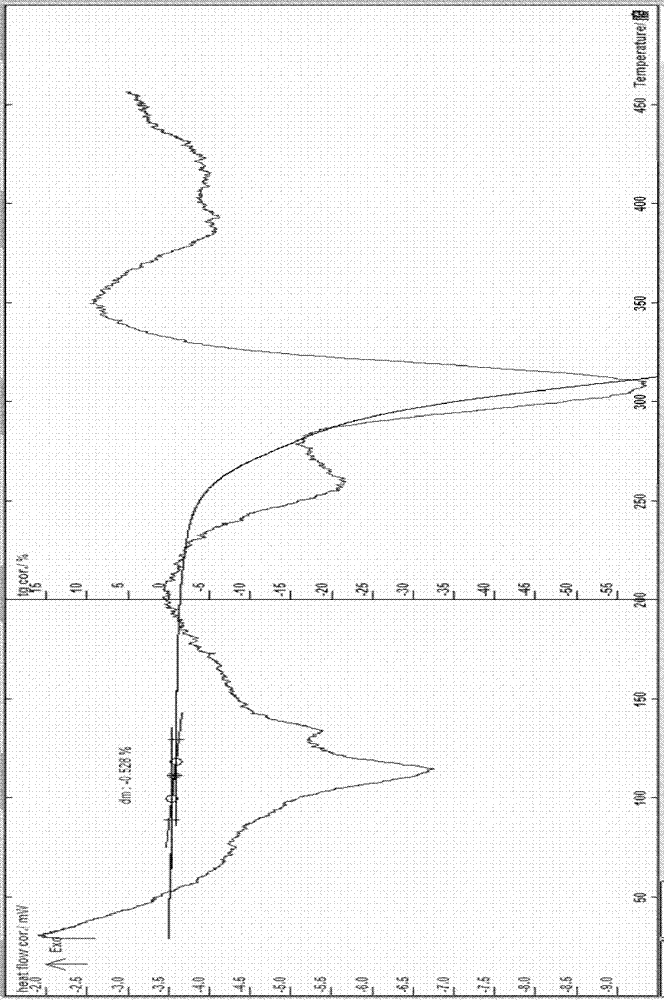
[0060] Example 3. The preparation of erythromycin ethylsuccinate 0.25 hydrate: at room temperature, in a 500ml three-necked flask, add 10 g of erythromycin ethylsuccinate 0.5 hydrate, add acetone to make it completely dissolve and slightly excessive, and stir Slowly add 50-70ml of pure water, cool to -10~4°C, let the precipitate precipitate out, filter, rinse the solid with water, filter to obtain a off-white solid, recrystallize with acetone and water, and filter with suction at about 60°C Vacuum drying at about 0.09MPa for about 16 hours yielded 6.7g of off-white crystals; melting point: 109-113°C (uncorrected); this sample was identified according to the identification items (1) and ( 2) Do the identification test, and the result is in line with the corresponding regulations; the water content measured by the Karl Fischer method is 0.68% (theoretical value is 0.52%), and the TG: weight loss is about 0.53% (see attached image 3 ) and the sample contains 0.25 crystal water w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com