Hexose ester, synthetic method thereof and use thereof
A six-carbon sugar ester and synthesis method technology, applied in the field of six-carbon sugar ester, can solve problems such as multi-drug resistance, difficulty in reaching the HIV virus reservoir, and inability to completely eliminate HIV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
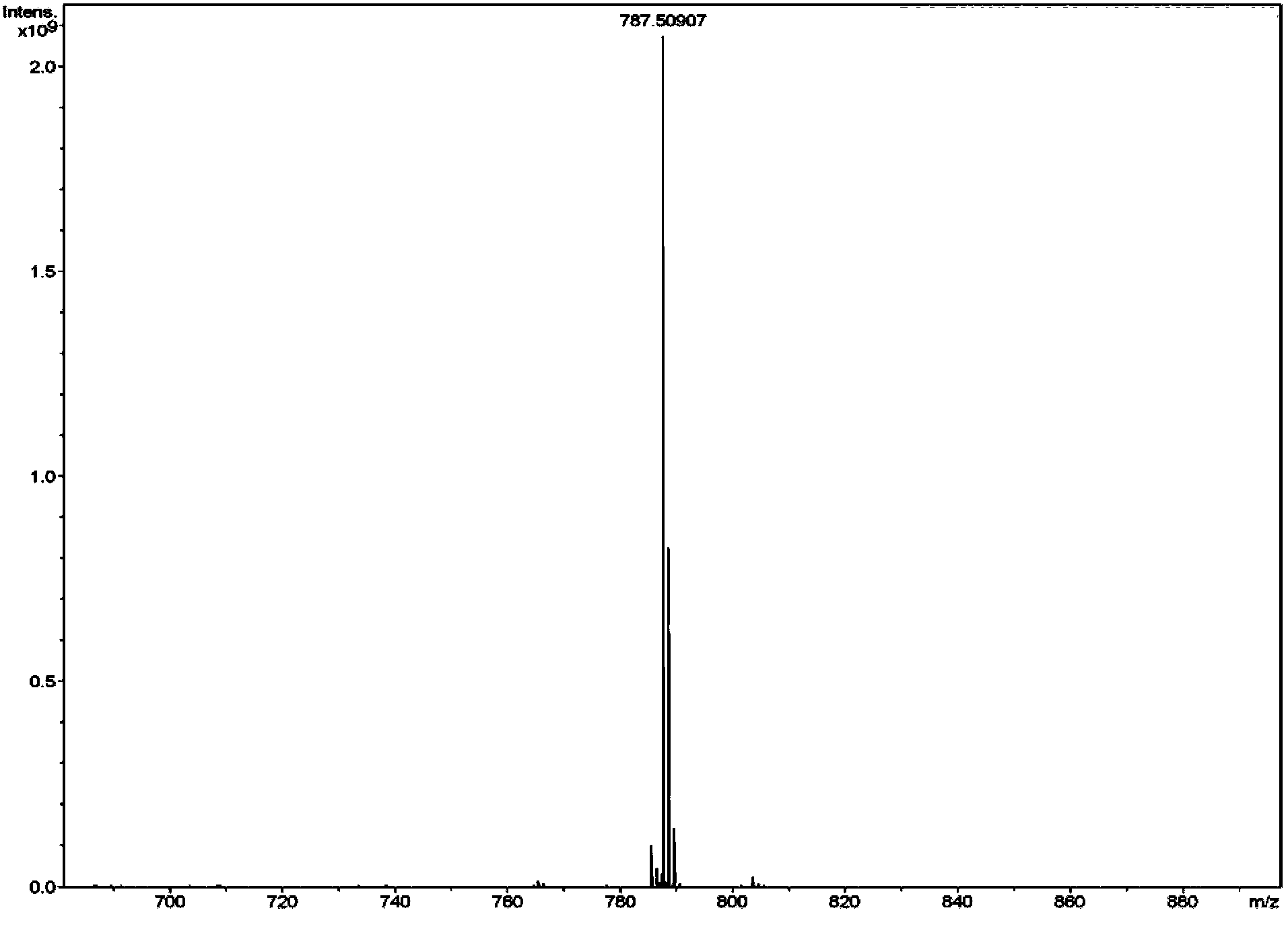
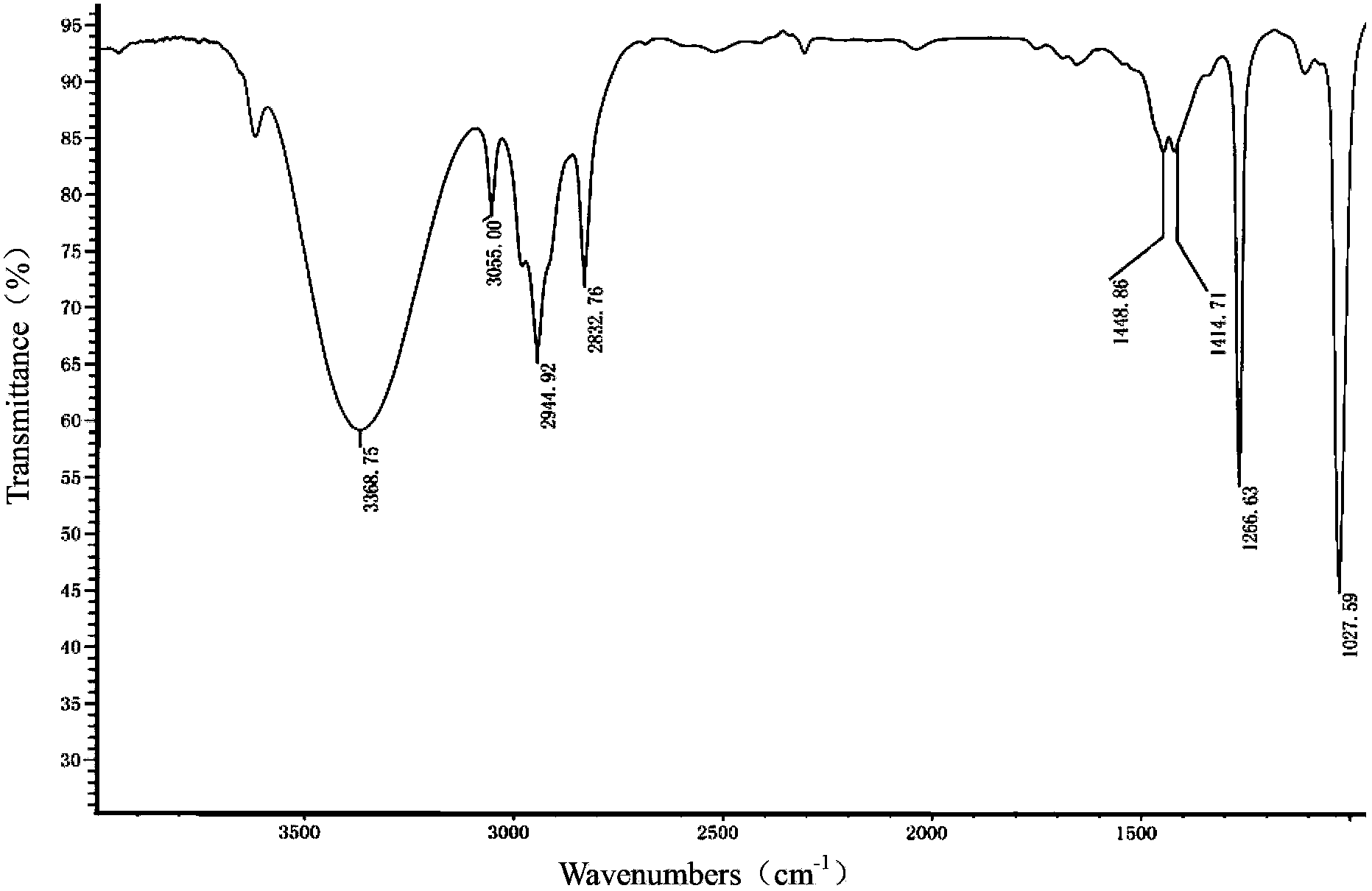
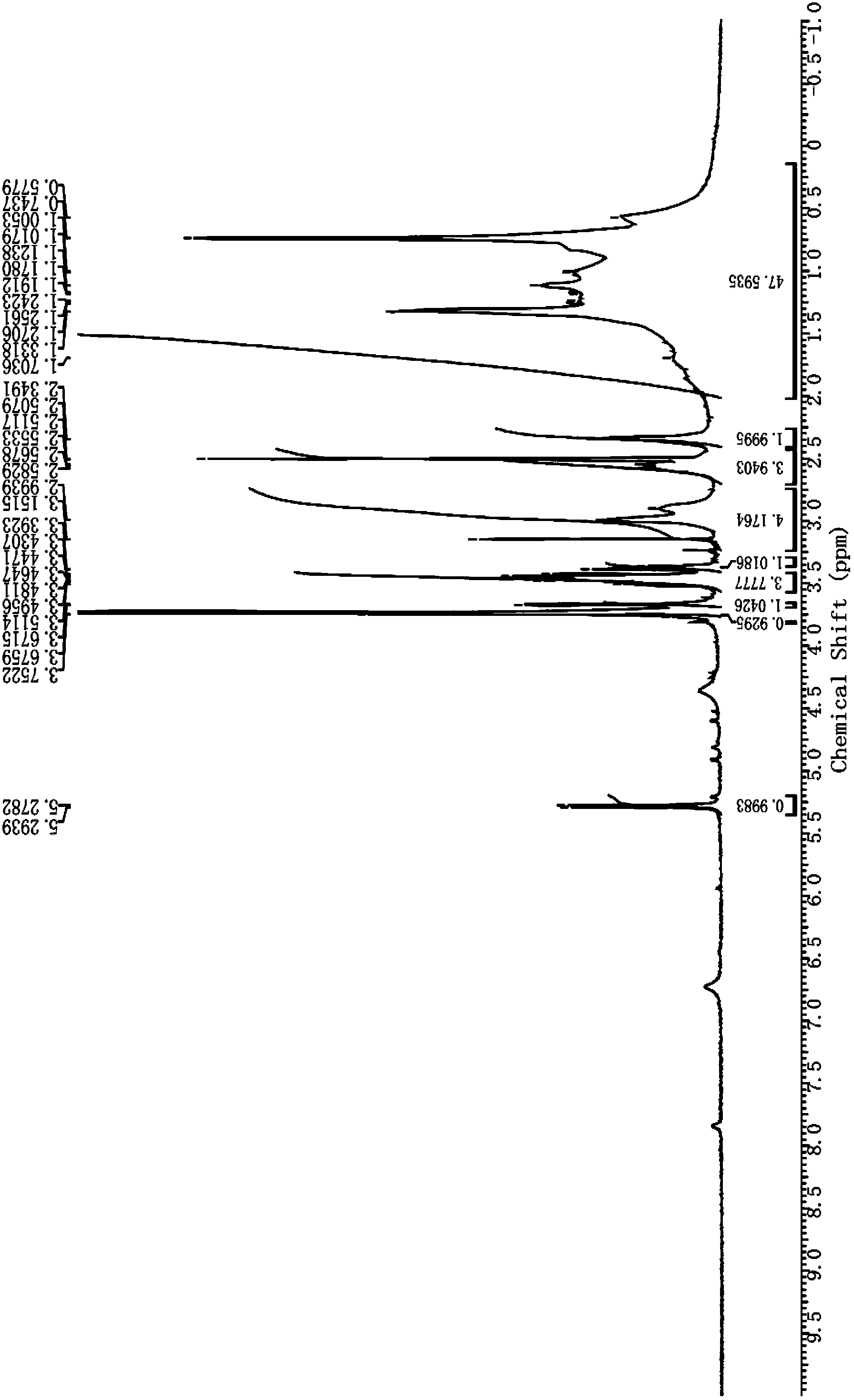
[0042] Embodiment 1 Preparation of galactose ester
[0043] 1. In N 2 Under protection, 1,4-butanediamine (4.47 g, 50.8 mol) was dissolved in 45 mL of anhydrous CH 2 Cl 2 In 20mL of anhydrous CH 2 Cl 2 Boc anhydride (2.77 g, 12.7 mmol) was added to the reaction liquid, and the ice bath was removed after 2 h of reaction, and stirred at room temperature for 16 h. Add 50mL CH 2 Cl 2 Diluted, the organic layer was washed successively with water (100mL), saturated NaCl solution (100mL), anhydrous NaCl 2 SO4 dry. The solvent was removed under reduced pressure with 0.5% NEt 3 CH 2 Cl 2 / MeOH=9 / 1 column chromatography to obtain colorless oil 1 (tert-butyl-(4-aminobutyl)carbamate, 2.15 g, yield 90%).
[0044] 2. In N 2 Under protection, compound 1 (1.88 g, 10 mmol) was dissolved in 40 mL of anhydrous CH 2 Cl 2 , add NEt under stirring in ice bath 3 (2.78 mL). Cholesterol chloroformate (4.93 g, 11 mmol) was dissolved in 10 mL of anhydrous CH 2 Cl 2 , slowly added dropw...
Embodiment 2
[0049] Example 2 Affinity of galactose ester-modified liposomes to macrophage membranes
[0050] (1) Preparation of fluorescent liposomes modified with galactose esters
[0051] Select doxorubicin (DOX) with a large conjugated system and excited fluorescence stability as a fluorescent probe, encapsulated in liposomes, and ammonium sulfate active drug loading method to prepare galactose ester modified DOX liposomes (DOX- Gal-L). Ordinary DOX liposome (DOX-L) does not contain galactose ester in the prescription.
[0052] (2) Affinity of galactose ester-modified liposomes to macrophage membranes
[0053] Take the RAW 264.7 cells in the logarithmic growth phase, adjust the concentration of the cell suspension, and adjust the cell concentration to 10 with the growth medium after counting the cells. 4 / mL, inoculate in 24-well plate, add 500 μL to each well, in 5% CO 2 After incubating at 37°C for 24 hours, 500 μL of free DOX, DOX-L, and DOX-Gal-L in a series of concentration gr...
Embodiment 3
[0054] Example 3 Biocompatibility of galactose esters
[0055] Take the RAW 264.7 cells in the logarithmic growth phase, adjust the concentration of the cell suspension, and adjust the cell concentration to 10 with the growth medium after counting the cells. 4 / mL, inoculate in 96-well plate, add 200 μL to each well, in 5% CO 2 After incubating at 37°C for 12 hours, a series of concentration-gradient galactose esters were added, and 6 wells were inoculated for each concentration. After continuing to culture for 24 hours, 20 μL of 0.5% MTT solution was added to each well, and cultured for 4 hours. Terminate the culture, and carefully aspirate the culture medium in the well. Add 100 μL of dimethyl sulfoxide to each well, shake on a shaker at low speed for 10 min, and fully dissolve the crystals. An enzyme-linked immunosorbent assay was used to measure the OD value at 570 nm of each well. At the same time set the zero well (medium, cells, dimethyl sulfoxide), control wells (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com