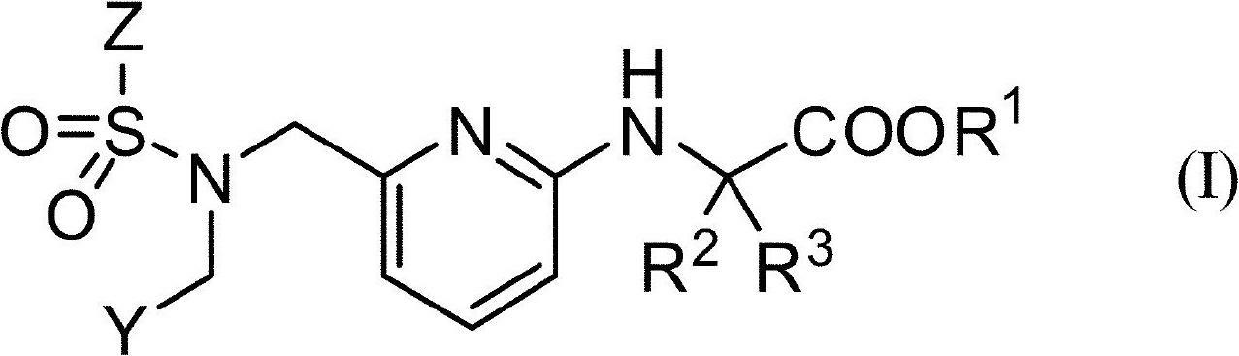
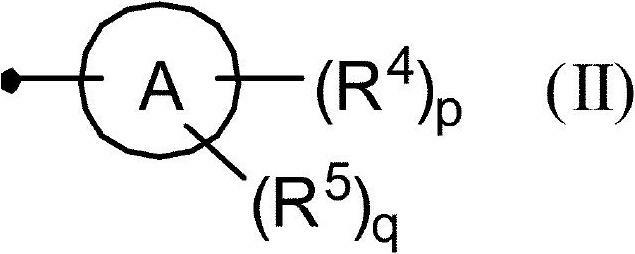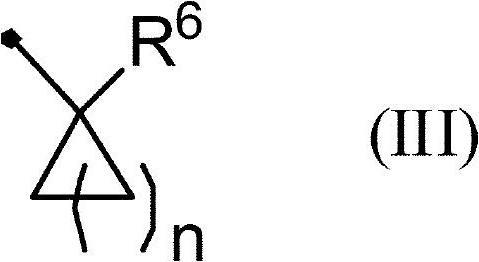Aminopyridine compound
A technology of aminopyridine and compounds, applied in the field of new aminopyridine compounds, can solve the problems of no specific disclosure, etc., and achieve the effect of excellent toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0776] (6-{[5-(1,1-dimethylpentyl)thiophen-2-ylmethyl](pyridin-3-ylsulfonyl base)aminomethyl}pyridin-2-ylamino)acetic acid (Exemplary Compound No. 673)
[0777] 1-(a): [tert-butoxycarbonyl (6-{[5-(1,1-dimethylpentyl)thiophene-2- Methyl](pyridin-3-ylsulfonyl)aminomethyl}pyridin-2-yl)amino]tert-butyl acetate
[0778](tert-butoxycarbonyl {6-[(pyridin-3-ylsulfonyl) aminomethyl] pyridin-2-yl} amino) tert-butyl acetate obtained in the same manner as Reference Example 1-(f) Add 1.27g (6.00mmol) of [5-(1,1-dimethylpentyl)thiophen-2-yl]methanol obtained in Reference Example 4-(c) to 4.0ml of tetrahydrofuran solution of 422mg (0.880mmol), 395 µl (1.60 mol) of tri-n-butylphosphine and 276 mg (1.60 mmol) of N,N,N',N'-tetramethylazodicarbonamide were stirred at room temperature for 16 hours. After completion of the reaction, water was added to the reaction solution, followed by extraction with ethyl acetate. The organic layer was washed with a saturated aqueous sodium chloride sol...
Embodiment 2
[0787] (6-{[5-(1,1-dimethylpentyl)thiophen-2-ylmethyl](pyridin-2-ylsulfonyl base)aminomethyl}pyridin-2-ylamino)acetic acid (Exemplary Compound No. 666)
[0788] 2-(a): [tert-butoxycarbonyl (6-{[5-(1,1-dimethylpentyl)thiophene-2- Methyl](pyridin-2-ylsulfonyl)aminomethyl}pyridin-2-yl)amino]tert-butyl acetate
[0789] 3.16 g (6.60 mmol) tetrahydrofuran 30ml solution, add 1.27g (6.00mmol) of [5-(1,1-dimethylpentyl)thiophen-2-yl]methanol obtained in Reference Example 4-(c), three-n- - 2.96ml (12.0mol) of butylphosphine and 2.07g (12.0mmol) of N,N,N',N'-tetramethylazodicarbonamide, stirred at room temperature for 16 hours. After completion of the reaction, water was added to the reaction solution, followed by extraction with ethyl acetate. The organic layer was washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was subjected to silica gel column chromatography (elution solvent; toluen...
Embodiment 3
[0798] (6-{(4-fluorobenzenesulfonyl)[5-(1,1-dimethylpentyl)thiophen-2-ylmethyl] Aminomethyl}pyridin-2-ylamino)acetic acid (Exemplary Compound No. 642)
[0799] 3-(a): [tert-butoxycarbonyl (6-{(4-fluorobenzenesulfonyl)[5-(1,1-dimethyl ylpentyl)thiophen-2-ylmethyl]aminomethyl}pyridin-2-yl)amino]tert-butyl acetate
[0800] 436 mg (0.880 mmol) of (tert-butoxycarbonyl {6-[(4-fluorobenzenesulfonyl) aminomethyl] pyridin-2-yl} amino) tert-butyl acetate obtained in Reference Example 3 in 4.0 ml of tetrahydrofuran Add 170mg (0.800mmol) of [5-(1,1-dimethylpentyl)thiophen-2-yl]methanol and 395μl of tri-n-butylphosphine (1.60 mol) and N, N, N', N'-tetramethyl azodicarbonamide 276 mg (1.60 mmol), stirred at room temperature for 16 hours. After completion of the reaction, water was added to the reaction solution, followed by extraction with ethyl acetate. The organic layer was washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate and concentrated und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com