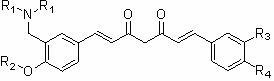
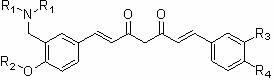
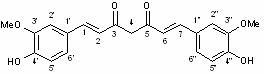
Nitrogen-containing substituent curcumin analogue and medical application thereof
A technology based on curcumin and nitrogen substitution, which is applied in the field of anti-tumor and neurodegenerative diseases, can solve the problems of non-absorption of curcumin after oral administration, limited medicinal development prospects, low bioavailability, etc., and achieves easy penetration through the blood-brain barrier. , mild inhibitory effect, efficient scavenging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1.3-dimethylaminomethyl-4-hydroxybenzaldehyde (compound 2a ) preparation
[0034]
[0035] 1.00 g (8 mmol) 4-hydroxybenzaldehyde, 1.12 g (8 mmol) dimethylamine in water (33%), 0.80 g (9.8 mmol) formaldehyde in water (37%) in 20 mL of methanol at 50 °C React overnight. The methanol was distilled off under reduced pressure, the residue was extracted with ethyl acetate (15 mL×3), the organic phases were combined, and washed with anhydrous Na 2 SO 4 Drying, concentration, and column chromatography using ethyl acetate / petroleum ether mixture as eluent gave 0.57 g of a light yellow oil, which solidified into a solid after standing. The yield was 39%, m.p.80~81°C. 1 H NMR (CDCl 3, 500MHz): δ 9.81 (s, 1H, C H O), 8.61 (s, 1H, O H ), 7.71-7.69 (m, 1H, aroma), 7.55-7.54 (d, 1H, aroma), 6.92-6.90 (d, 1H, aroma), 3.73 (s, 1H, C H 2 ), 2.36 (s, 6H, N(C H 3 ) 2 ); IR (KBr): v 3421.4 (O-H), 2957.6, 2863.6 (C-H, CH 3 ), 2729.5 (C-H, CHO), 1684.2 (C=O), 159...
Embodiment 2
[0036] Embodiment 2.5-hydroxyl-1-(3-dimethylaminomethyl-4-hydroxyl)-1,4-pentadien-3-one ( 3a ) preparation
[0037]
[0038] 0.79 g (7.92 mmol) acetylacetone and 0.50 g (7.18 mmol) B 2 o 3 Dissolve in ethyl acetate (5 mL), stir at 80°C for 30 min; add 0.65 g (3.6 mmol) of 2a and 0.34 g (1.48 mmol) (n-BuO) 3 B, continue to stir at 80°C for 30min; then add 0.10 g (1.43 mmol) of n-butylamine dropwise, stir at 100°C for 2h; then cool to 50°C, add 1N HCl aqueous solution (10mL), and stir for 30min. Cooled to room temperature, the reaction solution was extracted with ethyl acetate (25 mL×3), the organic phase was washed with saturated NaCl (15 mL×2), anhydrous NaCl 2 SO 4 After drying, column chromatography using ethyl acetate / petroleum ether mixture as eluent gave 0.13 g of a yellow solid with a yield of 14%, m.p.105-106°C. 1 HNMR (CDCl 3 , 500MHz): δ 15.53 (s, 1H, CH=C-O H ), 7.53-7.50 (d, 1H, arom-C H =CH), 7.38-7.36 (m, 1H, aroma), 7.14-7.14 (d, 1H, aroma), 6.83-6....
Embodiment 3
[0039] Example 3. 1-(3-Dimethylaminomethyl-4-hydroxyphenyl)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-1,4,6-pentanetri En-3-one ( 4a ) preparation
[0040]
[0041] 0.38 g (1.44 mmol) compound 3a and 0.15 g (2.13 mmol) B 2 o 3 Dissolve with ethyl acetate (5 mL). Add 4-hydroxy-3-methoxybenzaldehyde 0.30 g (2.00 mmol) and 0.49 g (2.11 mmol) (n-BuO) 3 5 mL ethyl acetate solution of B, stirred at 80°C for 30 min; added 0.04 g (0.51 mmol) n-butylamine, continued to stir at 80°C for 1 h; cooled to 50°C, added 0.4N HCl aqueous solution (5 mL), and stirred again 30min. Cooled to room temperature, the reaction solution was extracted with ethyl acetate (25 mL×3), washed with saturated NaCl (15 mL×3), anhydrous NaCl 2 SO 4 After drying, column chromatography using ethyl acetate / petroleum ether mixture as eluent gave 0.26 g of a yellow solid with a yield of 46%, m.p.158~160°C. 1 H NMR (CDCl 3 , 300Hz): δ 7.63-7.58 (d, 2H, CH=CH), 7.44-7.44 (m, 1H, aroma), 7.23-7.23 (d, 1H, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com