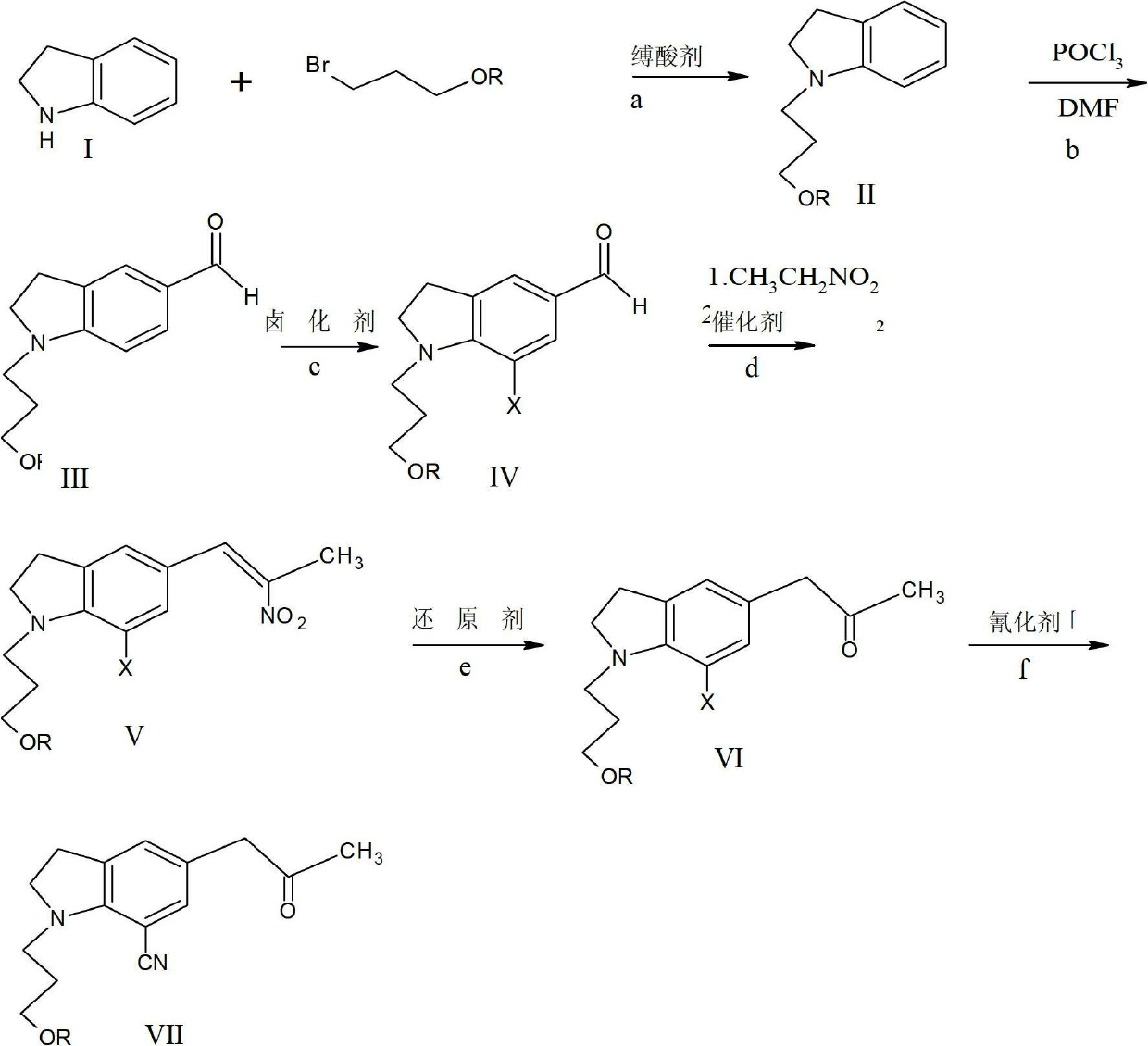
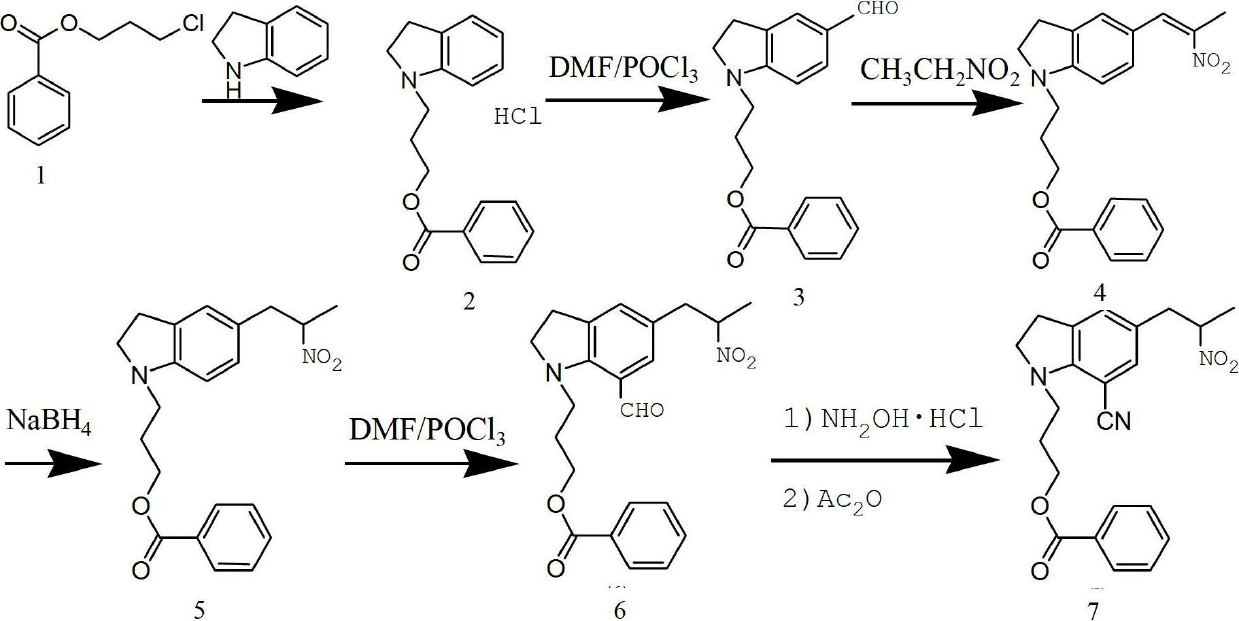
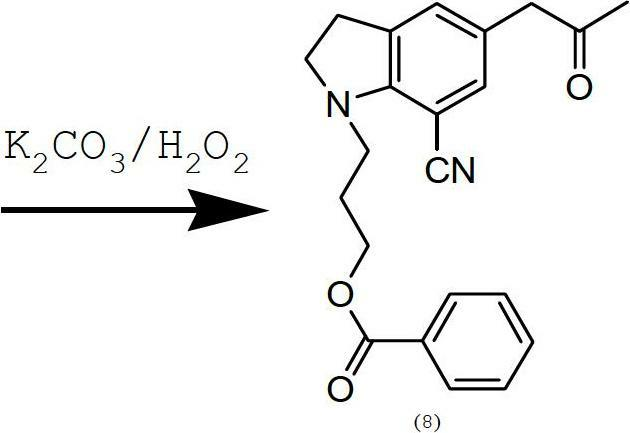
Preparing method of 1-(3-benzoyloxy propyl)-5-(2-oxopropyl)-7-indolinecarbonitrile
A technology of benzoyloxypropyl and cyanoindoline, which is applied in the field of preparation of 1--5--7-cyanoindoline, can solve the problem of unsuitability for industrial production, low total yield, There are many synthetic steps to achieve the effect of strong industrial application value, simplified reaction steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] Example 1: Put 48g (0.24mol) of 3-chloropropane benzoate, 24g (0.2mol) of indoline (ie compound I), 100ml of DMF, and 24.5g of triethylamine into the flask;
[0027] React at 100 degrees Celsius for 12 hours, and cool to room temperature;
[0028] Water was added to the reaction mixture, extracted with ethyl acetate, the organic layer was washed with water and saturated saline solution, dried with sodium sulfate, and the solvent was distilled off under reduced pressure;
[0029] Add 350ml of acetone to the residue, then add 20ml of hydrochloric acid dropwise with stirring, and stir overnight for crystallization to obtain 51g of compound II hydrochloride with a yield of 80% and a liquid phase purity of 98%.
example 2
[0030] Example two: drop 48g (0.24mol) of 3-chloropropane benzoate, 24g (0.2mol) of indoline (i.e. compound I) in a flask, 100ml of acetone, 26g of potassium carbonate, 2.4g of sodium iodide, and reflux React for 24 hours, cool to room temperature, add 200ml of acetone, filter, rinse with 50ml of acetone, transfer the filtrate to a flask, stir and add 20ml of hydrochloric acid dropwise, stir overnight for crystallization, and obtain 53g of the hydrochloride of compound II, yield It is 83%, and the liquid phase purity is 97%.
[0031] Alternatively, the protecting group can also be benzyl, that is, the reaction raw material is: 45g (0.24mol) of 3-chloropropaneanisole, and the rest remain unchanged. After the reaction, 47g of the hydrochloride of compound II is obtained, and the yield is 81, liquid The phase purity was 98%.
[0032] b. Preparation of 1-(3-benzoyloxypropyl)-5-formyl indoline, i.e. compound III:
[0033] Put 62.5ml of DMF into the reaction bottle, ice bath, add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com