Method for enzymatic synthesis of feruloyl oligosaccharide in mixed solvent
A technology of ferulic acid sugar ester and mixed solvent, which is applied in the field of enzymatic synthesis of ferulic acid sugar ester, can solve the problems of harsh conditions, poor control, cumbersome steps, etc., achieve mild reaction conditions, easy separation, and avoid reaction conditional effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
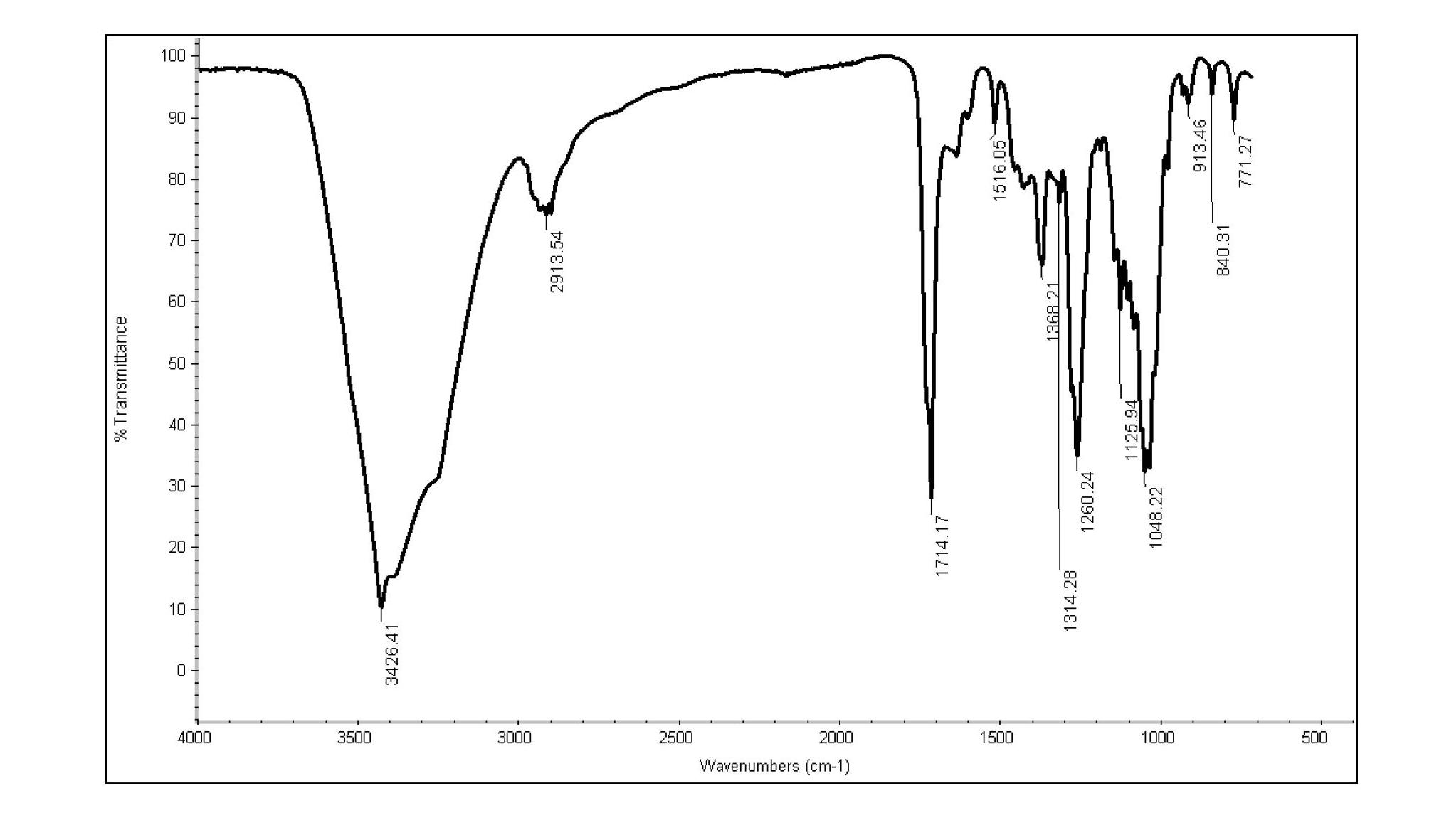
[0032] The synthesis of ferulic acid glucose ester catalyzed by different enzymes, the specific steps are as follows:
[0033] (1) Will After the molecule is activated and dried, it is added to the reaction solvent to be used, and the solvent is dried to obtain a dehydrated organic solvent after four days. The amount of molecular sieve used is 200 mg / ml.
[0034] (2) Enzymatic transesterification reaction: Take 3.8g of glucose and 1g of vinyl ferulic acid and add them to 100ml of dehydrated organic solvents of equal volume pyridine and tert-butanol, shake and dissolve, then mix, then add 2g of various enzymes, Alkaline protease produced by Bacillus subtilis (200,000, Wuxi Xuemei Technology Co., Ltd.); Lipase from Candida Cylindacea (5.18u / mg, sigma-Aldrich); Lipase from Candida rugosa (1104u / mg, sigma); Lipase from Rhizopus oryzae (55.7u / mg, sigma-Aldrich); Lipase Novozym 435 (7u / mg, Guangzhou Mingyuan Industry and Trade Co., Ltd.); Lipase from Pseudomonas fluorescens (2.2u / ...
Embodiment 2
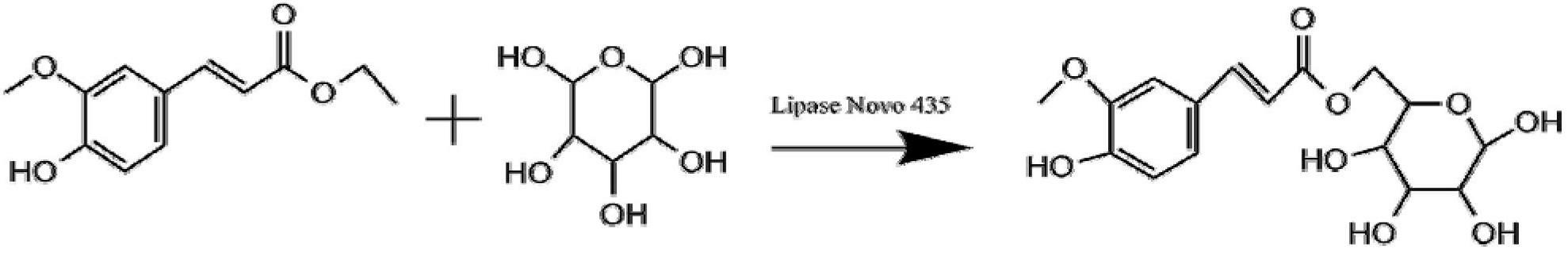
[0036] Different organic solvents catalyze the enzymatic synthesis of ferulic acid sugar ester, and the specific steps are as follows:
[0037] (1) Will After molecular sieve activation 4 is dried, it is added to the reaction solvent to be used, and the solvent is dried to obtain a dehydrated organic solvent after four days. The amount of molecular sieve used is 200 mg / ml.
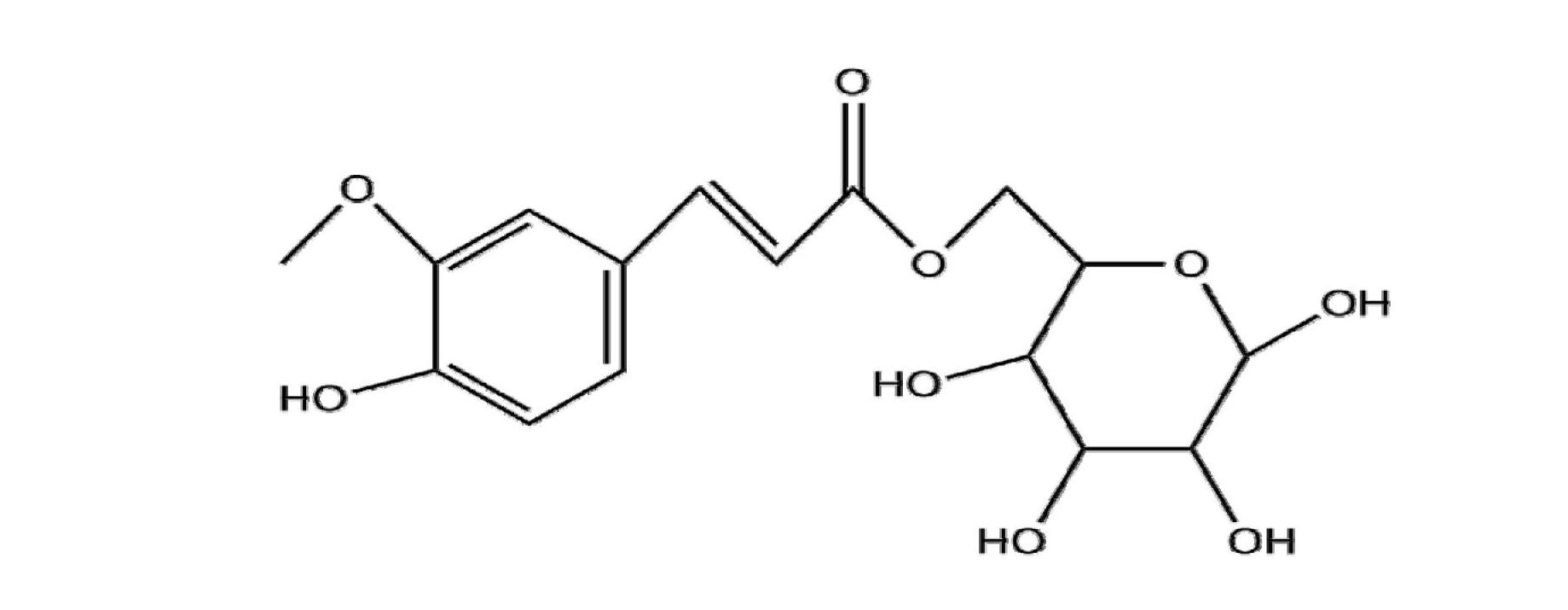
[0038] (2) Enzymatic transesterification reaction: Add 3.8g~58g of glucose and 1g of ferulic acid to 100ml of different dehydrated organic solvents pyridine, tert-butanol, and 100ml of equal volumes of mixed solvents of tert-butanol and pyridine, and then add 2g of lipase Novozyon 435 (7u / mg); add 20g of molecular sieves, shake at 220r / min for 72h at 50°C. After the reaction is finished, the molecular sieve and enzyme are removed by filtering with filter paper, and the obtained filtrate is evaporated by a rotary evaporator to remove the organic solvent, and then purified by a silica gel column to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com