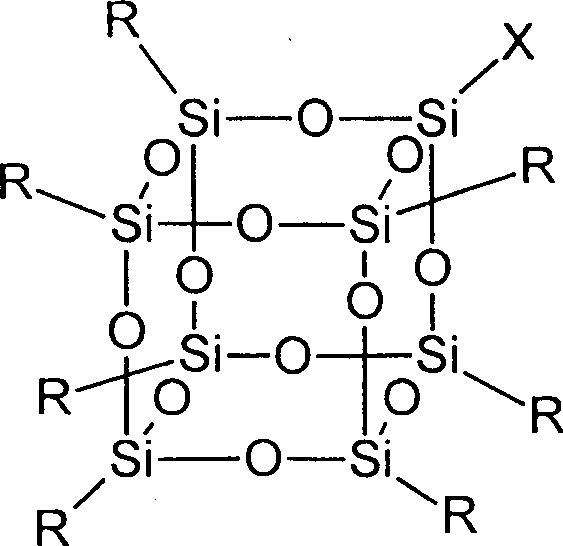
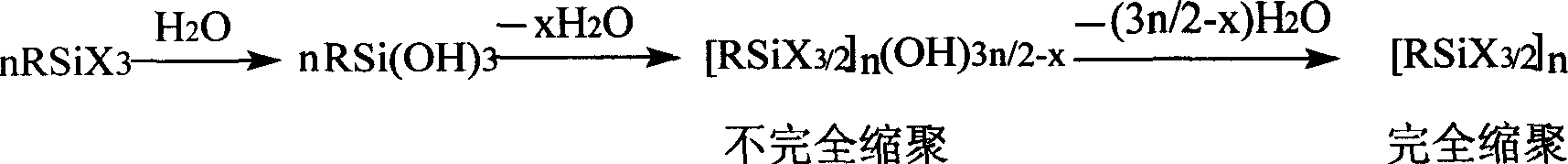Synthesis method for substituting sesquialter siloxane by non-functional alkyl
A polysilsesquioxane and a synthesis method technology, applied in the direction of silicon organic compounds and the like, can solve the problems of restricting the research, popularization and application of POSS compounds, complicated separation and purification processes, and high synthesis cost, and achieve good product structure unity, The process is simple and easy to implement, and the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
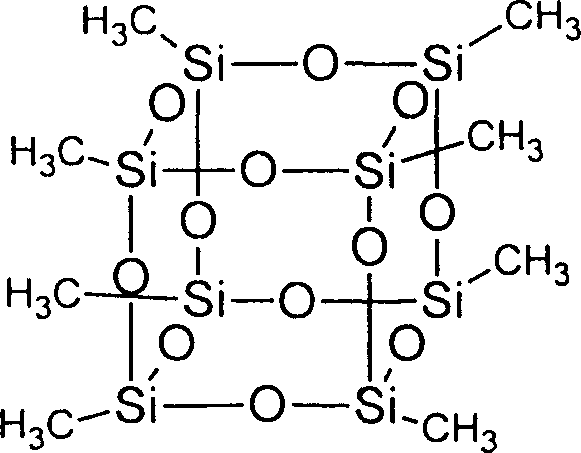
[0016] Embodiment 1: the synthesis of the clathrate octapolysilsesquioxane of methyl substitution
[0017] A 500ml three-neck flask was placed in a constant temperature water bath at 80°C, and 90g (5mol) of deionized water, 40ml of acetone, 10ml of acetonitrile, 10ml (about 0.1mol) of triethylamine, and 0.5ml of a mixture of concentrated hydrochloric acid and formic acid with a volume ratio of 1:1 were sequentially Add to the three-necked flask, stir to make it evenly mixed. Then, 178 g (1 mol) of methyltriethoxysilane was added dropwise to the mixed solution, and stirred at 80° C. for 18 h at constant temperature under reflux. The crude product in the form of light yellow powder was obtained by filtration. The crude product was washed three times with 1000 ml of deionized water, then washed with acetone, and dried in vacuum to obtain the final product in the form of white powder with a yield of 86.0%. The molecular structural formula is as follows:
[0018]
Embodiment 2
[0019] Example 2: Synthesis of methyl-substituted cage octapolysilsesquioxane
[0020] Place a 500ml three-neck flask in a water bath with a constant temperature of 50°C, add 180g (10mol) of deionized water, 67ml of acetone, 33ml of acetonitrile, 30ml (about 0.3mol) of trimethylamine, and 5ml of a mixture of concentrated hydrochloric acid and acetic acid with a volume ratio of 1:3. To the three-necked flask, stir to make it evenly mixed. Then 178 g (1 mol) of methyltriethoxysilane was added dropwise to the mixed solution, and stirred under constant temperature at 50° C. for 18 h under reflux. Filter to obtain a light yellow powdery crude product, first wash the crude product with 1000ml deionized water for 3 times, then wash with acetone, and vacuum dry to obtain a white powdery final product. Yield 88.2%.
Embodiment 3
[0021] Embodiment 3: Synthesis of cyclopentyl-substituted clathrate octasilsesquioxane
[0022] Place a 500ml three-neck flask in a constant temperature water bath at 70°C, mix 144g (8mol) of deionized water, 35ml of methyl ethyl ketone, 15ml of butyronitrile, 12ml (about 0.2mol) of trimethylamine, and 2ml of concentrated hydrochloric acid and acetic acid at a volume ratio of 1:2 The solution was added into the three-necked flask in turn, and stirred to make it evenly mixed. Then 232 g (1 mol) of cyclopentyltriethoxysilane was added dropwise to the mixed solution, and stirred under constant temperature at 70° C. for 18 h under reflux. The solvent was removed under vacuum at room temperature to obtain the crude product as light yellow powder. The crude product was first washed with 1000 ml of deionized water for 3 times, then washed with acetone, and vacuum-dried to obtain a white powdery final product with a yield of 85.5%. The molecular structural formula is as follows:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com