Preparation method of lidocaine ethosome
A lidocaine and alcohol plastid technology is applied in the field of preparation of lidocaine alcohol plastids, can solve the problems of low encapsulation rate and the like, and achieves the effects of high encapsulation rate, easy industrialization and simple prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: The preparation method of lidocaine ethosome of this embodiment comprises the following steps:
[0021] (1) Add 0.3 g of lecithin and 0.1 g of lidocaine into 4.5 ml of ethanol with a volume fraction of 30%, stir to make it dissolve initially, and ultrasonically induce dissolution until a uniform milky white suspension is formed;
[0022] (2) Transfer the above suspension into a round-bottomed flask, seal the mouth of the bottle, place it on a magnetic stirrer, set the temperature to 30°C, and the rotation speed to 25r / min, stir evenly under airtight conditions, and stir in a trickle while stirring. Inject 6.5ml of PBS buffer solution with pH 7.0, and then continue to stir evenly;
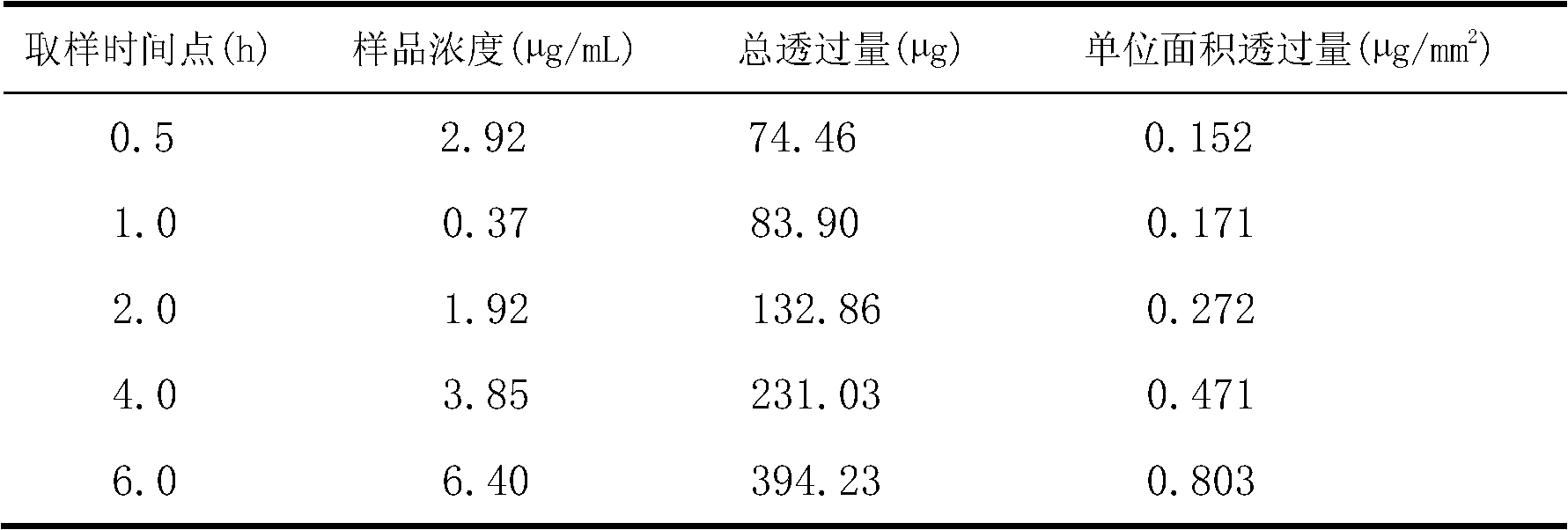
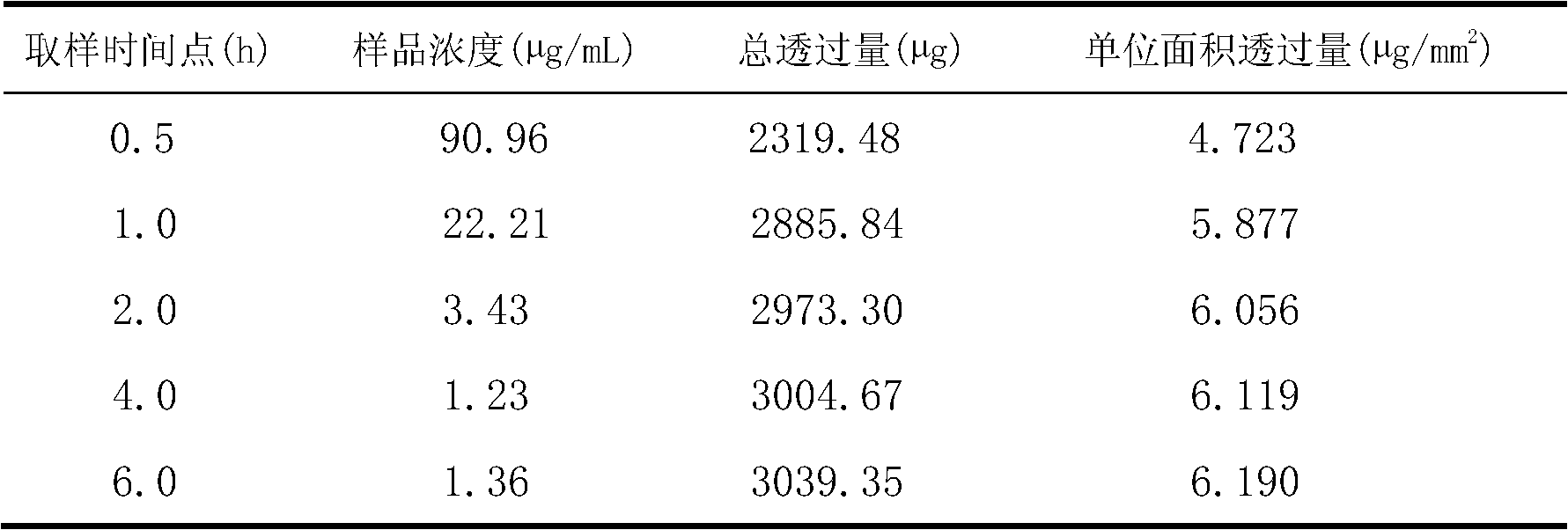
[0023] (3) Ultrasonic treatment for 1 min under ice bath conditions (ultrasonic power 200W, ultrasonic 5s, stop 3s), and finally filter, the filter membrane pore size is 0.40μm, and lidocaine ethosomes are obtained.
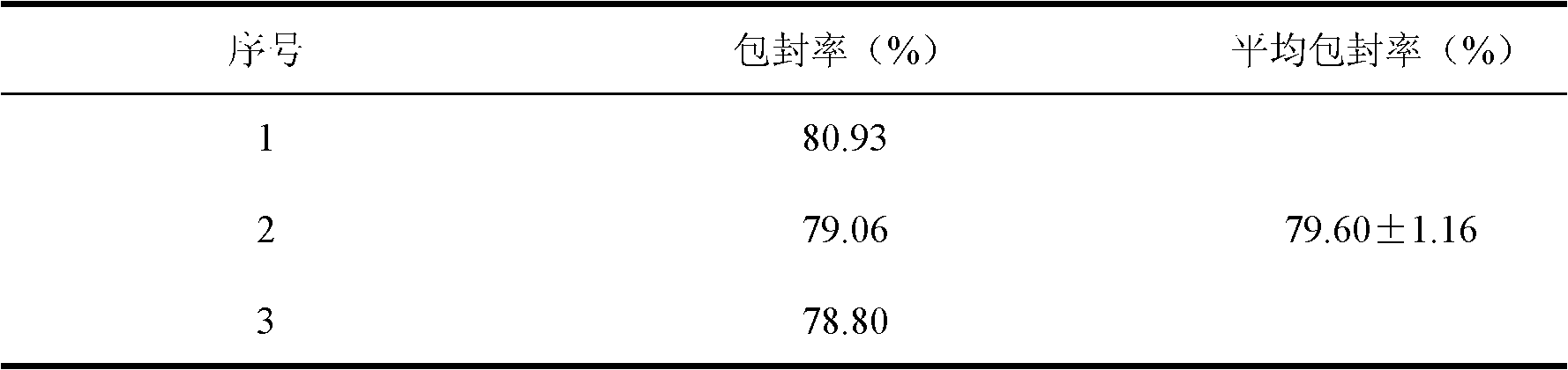
[0024] Determination of encapsulation efficiency: put 10 mL ...
Embodiment 2
[0026] Embodiment 2: The preparation method of lidocaine ethosome of this embodiment comprises the following steps:
[0027] (1) Add 0.3g lecithin and 0.1g lidocaine into 4.5ml of ethanol with a volume fraction of 40%, stir to make it dissolve initially, and ultrasonically induce dissolution until a uniform milky white suspension is formed;
[0028] (2) Transfer the above suspension into a round-bottomed flask, seal the neck of the bottle, place it on a magnetic stirrer, set the temperature to 35°C, and the rotation speed to 30r / min, stir evenly under airtight conditions, and stir in a trickle while stirring. Inject 6.5ml of PBS buffer solution with pH 7.0, and then continue to stir evenly;
[0029] (3) Ultrasonic treatment for 2 minutes under ice-bath conditions (ultrasonic power 200W, ultrasonic 5s, stop 3s), and finally filter, the filter membrane pore size is 0.45μm, to obtain lidocaine ethosome.
[0030] Measuring encapsulation efficiency: method is the same as embodimen...
Embodiment 3
[0032] Embodiment 3: The preparation method of lidocaine ethosome of this embodiment comprises the following steps:
[0033] (1) Add 0.3 g of lecithin and 0.1 g of lidocaine into 4.5 ml of ethanol with a volume fraction of 50%, stir to make it dissolve initially, and ultrasonically induce dissolution until a uniform milky white suspension is formed;
[0034] (2) Transfer the above suspension into a round-bottomed flask, seal the bottle mouth, place it on a magnetic stirrer, set the temperature to 40°C, and the rotation speed to 35r / min, stir evenly under airtight conditions, and stir in a trickle while stirring. Inject 6.5ml of PBS buffer solution with pH 7.0, and then continue to stir evenly;
[0035] (3) Ultrasonic treatment for 3 minutes under ice-bath conditions (ultrasonic power 200W, ultrasonic 5s, stop 3s), and finally filter, the pore size of the filter membrane is 0.50 μm, to obtain lidocaine ethosome.
[0036] Measuring encapsulation efficiency: method is the same a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Membrane pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com