Production method of triple inactivated vaccine for newcastle disease, infectious bursal disease and viral arthritis
A technology for bursal disease and chicken Newcastle disease, applied in antiviral agents, viral antigen components, pharmaceutical formulations, etc., can solve the problems of uncontrollable epidemic development, high mortality rate, slow growth, etc., to reduce the occurrence of epidemics and The effect of spreading, rapid antibody production, and long protection period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
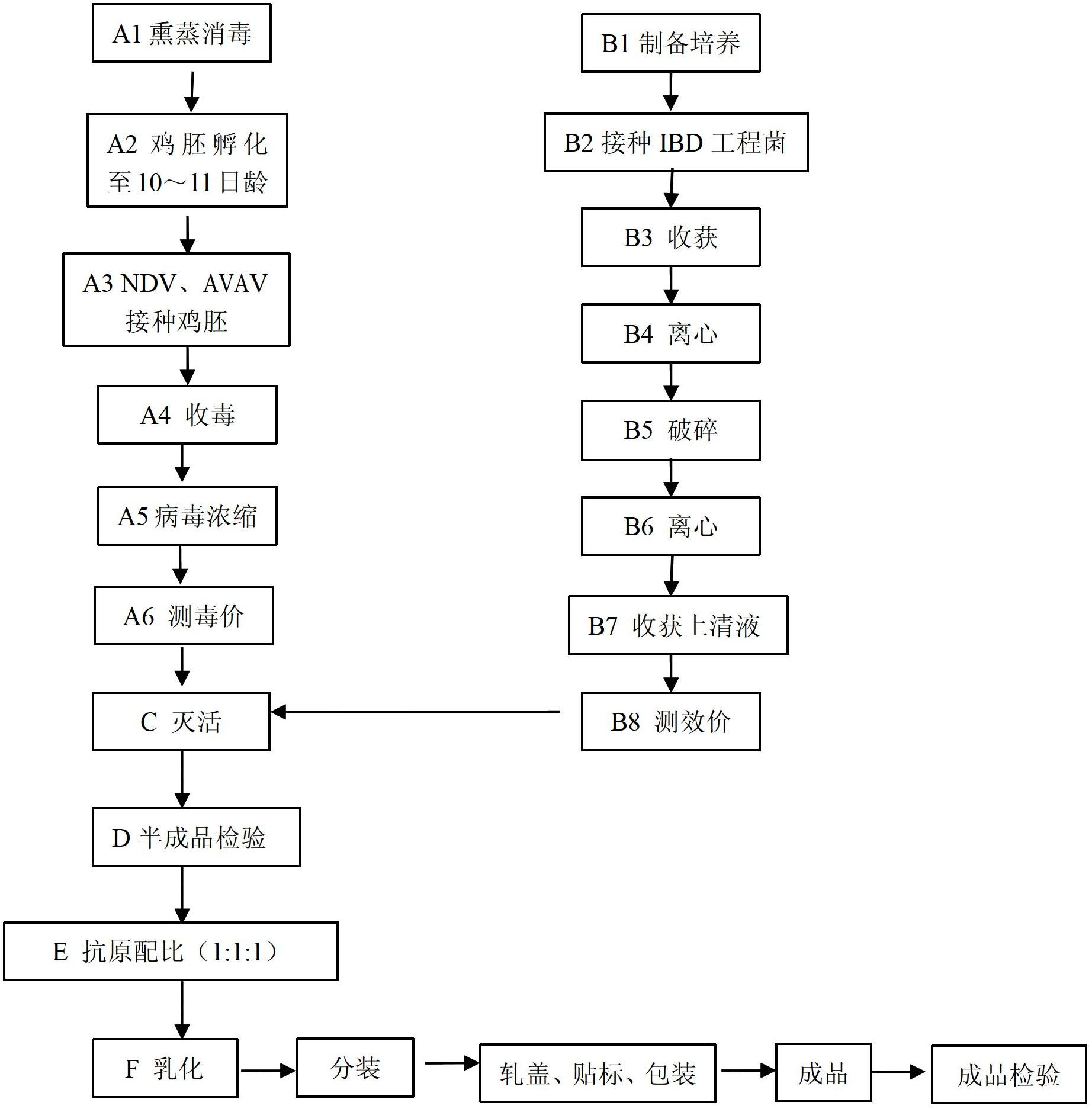
Embodiment 1
[0086] (1) Antigen preparation and inspection of semi-finished products:
[0087] 1. Preparation of Chicken Newcastle Disease Virus Liquid
[0088] (1) Inoculate the virus seeds used for production, and use sterilized saline to make 10 -4 Dilute and inoculate 200 10-day-old susceptible chicken embryos, 0.1ml per embryo, seal the pinholes after inoculation, and continue to incubate at 37°C without turning the embryos.
[0089] (2) Incubation and observation After the chicken embryos were inoculated, the embryos were photographed once a day, and 11 chicken embryos that died 60 hours ago were discarded. After that, the embryos were photographed once every 4-6 hours, and the dead embryos were taken out at any time until 96 hours, a total of 155 dead embryos were taken out, and all of them were taken out, with the air chamber upward, and cooled at 2-8°C for 12 hours.
[0090] (3) Harvesting Take out the cooled chicken embryos and harvest the allantoic fluid of the chicken embryo...
Embodiment 2
[0123] Finished product inspection - the laboratory trial-produced a batch of triple inactivated vaccine about 6000ml, the batch number is 2010001.
[0124] 1. character
[0125] Appearance Milky white emulsion.
[0126] Dosage form water-in-oil type. Take a clean straw, suck a small amount of vaccine and drop it into cold water, except for the first drop, it will not spread.
[0127] Stability Take 10ml of the vaccine and add it to a centrifuge tube, centrifuge at 3000r / min for 15 minutes, and the water phase precipitated at the bottom of the tube is 0.1ml.
[0128] The viscosity is carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and the viscosity is 52cP, which meets the regulations.
[0129] 2. Filling volume inspection According to the appendix of the current "Chinese Veterinary Drug Code", the results of the measurement of the three bottles were 251.3ml, 252.1ml, and 251.5ml, which met the regulations.
[0130] 3. Sterility te...
Embodiment 3
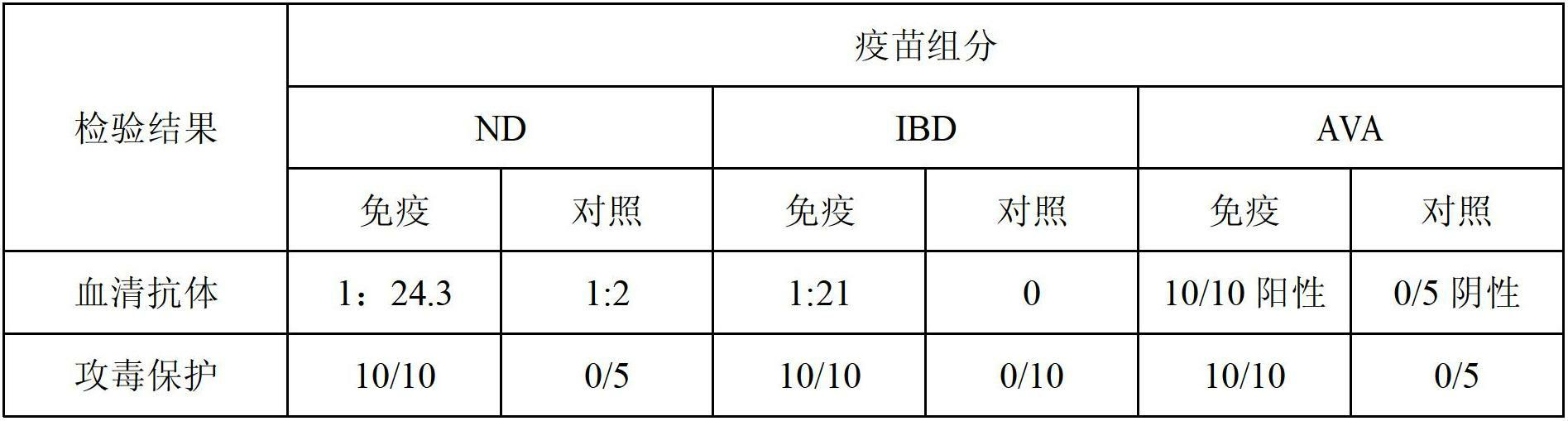
[0147] We have carried out a comparative test of the immune effect of the triple vaccine and each component single vaccine (commercialized product produced by the inventor's company), the test method is as follows:
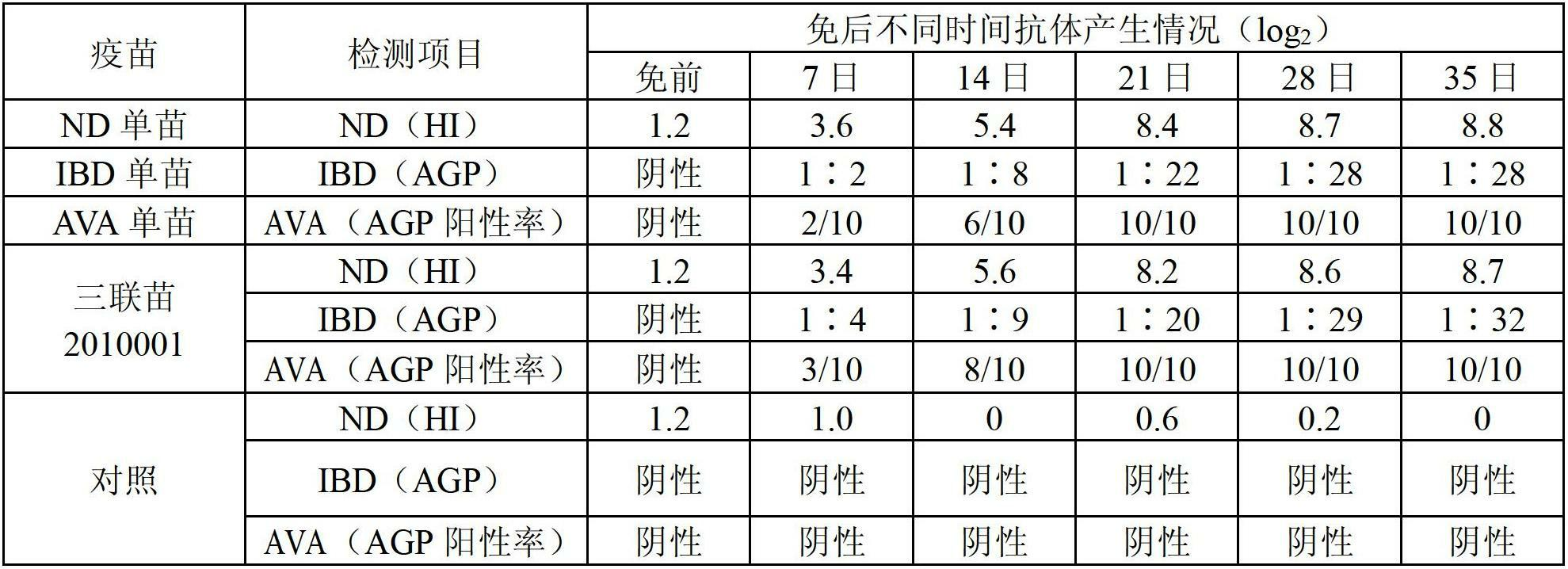
[0148] 50 21-day-old SPF chickens were used, and 10 chickens were immunized with triple vaccine, Newcastle disease, infectious bursal disease, and viral arthritis single vaccine, and 0.5ml of vaccine was injected subcutaneously per chicken, and the other 10 chickens were not immunized as a control. Breeding in groups. Chickens in each group were blood collected before immunization and 7, 14, 21, 28, 35 days after immunization, serum was separated, and serum antibodies were measured. The results are compared in Table 2 below.
[0149] Table 2 The results of the comparative test of the immune effect of the triple vaccine and the single vaccine of each component
[0150]
[0151] Note:
[0152] Bacterial endotoxin test by gel method
[0153] Pyrosate Endotoxin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com