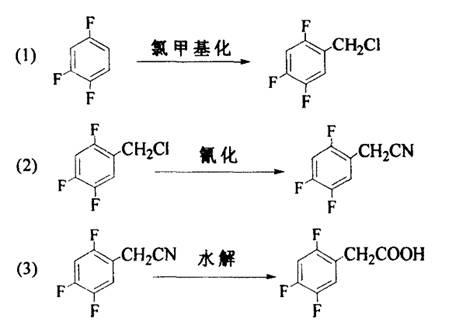
Preparation methods of 2, 4, 5-trifluoro-benzyl chloride and 2, 4, 5-trifluoro-phenylacetic acid
A technology of trifluorophenylacetic acid and trifluorobenzyl chloride, applied in the field of medicine and chemical industry, can solve the problems of high waste liquid waste treatment cost and large amount of three wastes, and achieve the effects of less amount of three wastes, low treatment cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of 2,4,5-trifluorobenzyl chloride
[0025] Under normal temperature and pressure, hydrogen chloride gas is passed into 500ml of 80% sulfuric acid solution for 2-3 hours to obtain a saturated hydrogen chloride solution.
[0026] Add 66 grams (0.5 mol) of 1,2,4-trifluorobenzene and 18.8 grams of paraformaldehyde (equivalent to 0.62 mol monomer formaldehyde) into a 250ml four-necked reaction flask, and add the prepared saturated hydrogen chloride dropwise at 30°C Sulfuric acid solution 90ml, after the dropwise addition, stirred and reacted for 1-2 hours, poured into ice water, separated the organic layer, washed with water until neutral, dried and distilled under reduced pressure to obtain 82 grams of 2,4,5-trifluorobenzyl chloride, Content 99.4%, yield 91.1%.
[0027] 1 H NMR (300MHz, CDCl 3 ) δppm: 4.51 (2H, s, CH 2 Cl) 6.32 (4H, s, 2 CH 2 ).
[0028] MS (FAB, m / z): 195.5 (M + +1).
Embodiment 2
[0029] Example 2 Preparation of 2,4,5-trifluorobenzyl chloride
[0030] Under normal temperature and pressure, hydrogen chloride gas is passed into 500ml of 90% sulfuric acid solution for 2-3 hours to obtain a saturated hydrogen chloride solution.
[0031] Add 66 grams (0.5 mol) of 1,2,4-trifluorobenzene and 18.8 grams of paraformaldehyde (equivalent to 0.62 mol of monomeric formaldehyde) into a 500ml four-necked reaction flask, and add the prepared saturated hydrogen chloride dropwise at 20°C Sulfuric acid solution 190ml, stirred and reacted for 1-2 hours after the dropwise addition, poured into ice water, separated the organic layer, washed with water until neutral, dried and distilled under reduced pressure to obtain 85 grams of 2,4,5-trifluorobenzyl chloride, Content 99.2%, yield 94.4%.
Embodiment 3
[0032] Example 3 Preparation of 2,4,5-trifluorobenzyl chloride
[0033] Under normal temperature and pressure, hydrogen chloride gas is passed into 500ml of 95% sulfuric acid solution for 2-3 hours to obtain a saturated hydrogen chloride solution.
[0034] Add 66 grams (0.5 mol) of 1,2,4-trifluorobenzene and 18.8 grams of paraformaldehyde (equivalent to 0.62 mol of monomeric formaldehyde) into a 500ml four-necked reaction flask, and add the prepared saturated hydrogen chloride dropwise at 0°C 260ml of sulfuric acid solution, after the dropwise addition, stirred and reacted for 1-2 hours, poured into ice water, separated the organic layer, washed with water until neutral, dried and distilled under reduced pressure to obtain 83 grams of 2,4,5-trifluorobenzyl chloride, Content 99.5%, yield 92.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com