Preparation method of gene and hydrophobic drug co-supported PEG (polyethyleneglycol) nanoparticles
A hydrophobic drug and nanoparticle technology, which is applied in gene therapy, drug combination, pharmaceutical formulation and other directions, can solve problems such as affecting gene transfection efficiency, reducing the circulation time of gene delivery system, etc., and achieves simple and easy preparation method, easy operation, and easy loading capacity. The effect of adjusting and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The steps of a method for preparing PEGylated nanoparticles co-loaded with genes and hydrophobic drugs are as follows:
[0021] 1) Prepare a β-cyclodextrin modified polycation solution with a concentration of 0.5-4 mg / mL;
[0022] 2) Prepare ferrocene-modified polyethylene glycol solution with a concentration of 0.5-4 mg / mL;
[0023] 3) Mix the solutions of step 1) and step 2) so that the molar ratio of β-cyclodextrin to ferrocene is 4:1-2:1, and then stand still after ultrasonication for 30 min;
[0024] 4) Prepare a hydrophobic drug solution with a concentration of 2 mg / mL;
[0025] 5) Add the hydrophobic drug solution prepared in step 4) dropwise to the solution in step 3), so that the molar ratio of β-cyclodextrin to hydrophobic drug is 1:1~1:4, stir in the dark for 24 hours, and dialyze for 8~ 24h, lyophilized to obtain β-cyclodextrin modified polycation / ferrocene modified polyethylene glycol / hydrophobic drug inclusion compound powder;
[0026] 6) Prepare β-cycl...
Embodiment 1
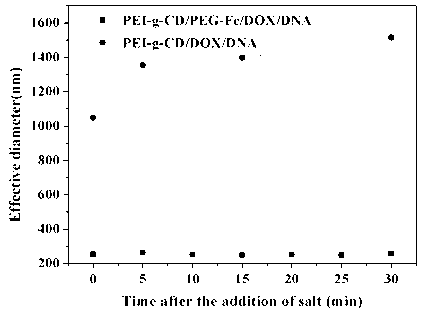
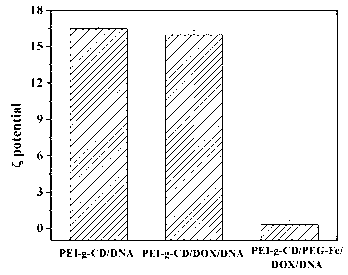
[0031] Prepare a β-cyclodextrin-modified polyethyleneimine (PEI-g-CD) solution with a concentration of 4 mg / mL and a ferrocene-modified polyethylene glycol (PEG-Fc) solution with a concentration of 4 mg / mL. The two solutions were mixed according to the molar ratio of β-CD and PEG-Fc in PEI-g-CD at a ratio of 2:1, sonicated for 30 min and left to stand for use. Add 2 mg / mL doxorubicin (DOX) dimethyl sulfoxide solution dropwise to the above mixed solution so that the molar ratio of CD and DOX in PEI-g-CD is 1:1, and stir in the dark for 24 h, then dialyzed for 8 h to 24 h, and freeze-dried to obtain PEI-g-CD / PEG-Fc / DOX clathrate powder. The PEI-g-CD / PEG-Fc / DOX clathrate powder prepared as above was used to measure the drug loading and encapsulation efficiency of doxorubicin (DOX) by ultraviolet-visible spectrophotometer (UV). The content was 9%, and the encapsulation efficiency was 57%, which indicated that β-cyclodextrin modified polyethyleneimine could effectively include the...
Embodiment 2
[0034] Prepare a solution of β-cyclodextrin-modified polylysine (PLL-g-CD) with a concentration of 2 mg / mL and a solution of ferrocene-modified polyethylene glycol (PEG-Fc) with a concentration of 2 mg / mL. The two solutions were mixed according to the molar ratio of β-CD and PEG-Fc in PLL-g-CD as 3:1, and then left to stand after ultrasonication for 30 min. Add 2 mg / mL paclitaxel (PTX) chloroform solution dropwise to the above mixed solution, so that the molar ratio of CD and PTX in PLL-g-CD is 1:2, stir for 24 h in the dark, and then dialyze for 8 h ~24 h, lyophilized to obtain PLL-g-CD / PEG-Fc / PTX clathrate powder. Prepare a PLL-g-CD / PEG-Fc / PTX solution with a concentration of 0.5 mg / mL and a p16 (multiple tumor suppressor 1 gene) solution with a concentration of 75 μg / mL. The above-mentioned PLL-g-CD / PEG-Fc / PTX solution was mixed with 250 μL DNA solution in equal volume by vortexing and allowed to stand for 30 min to prepare PEGylated nanoparticles co-loaded with genes and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com