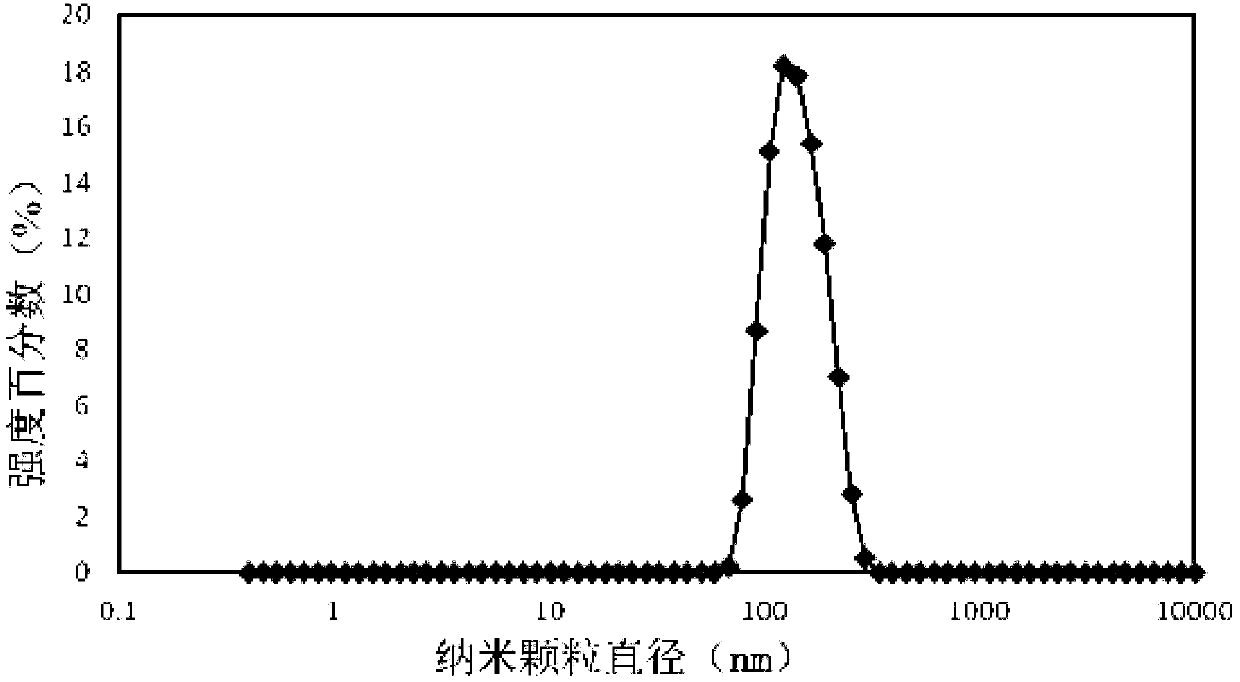
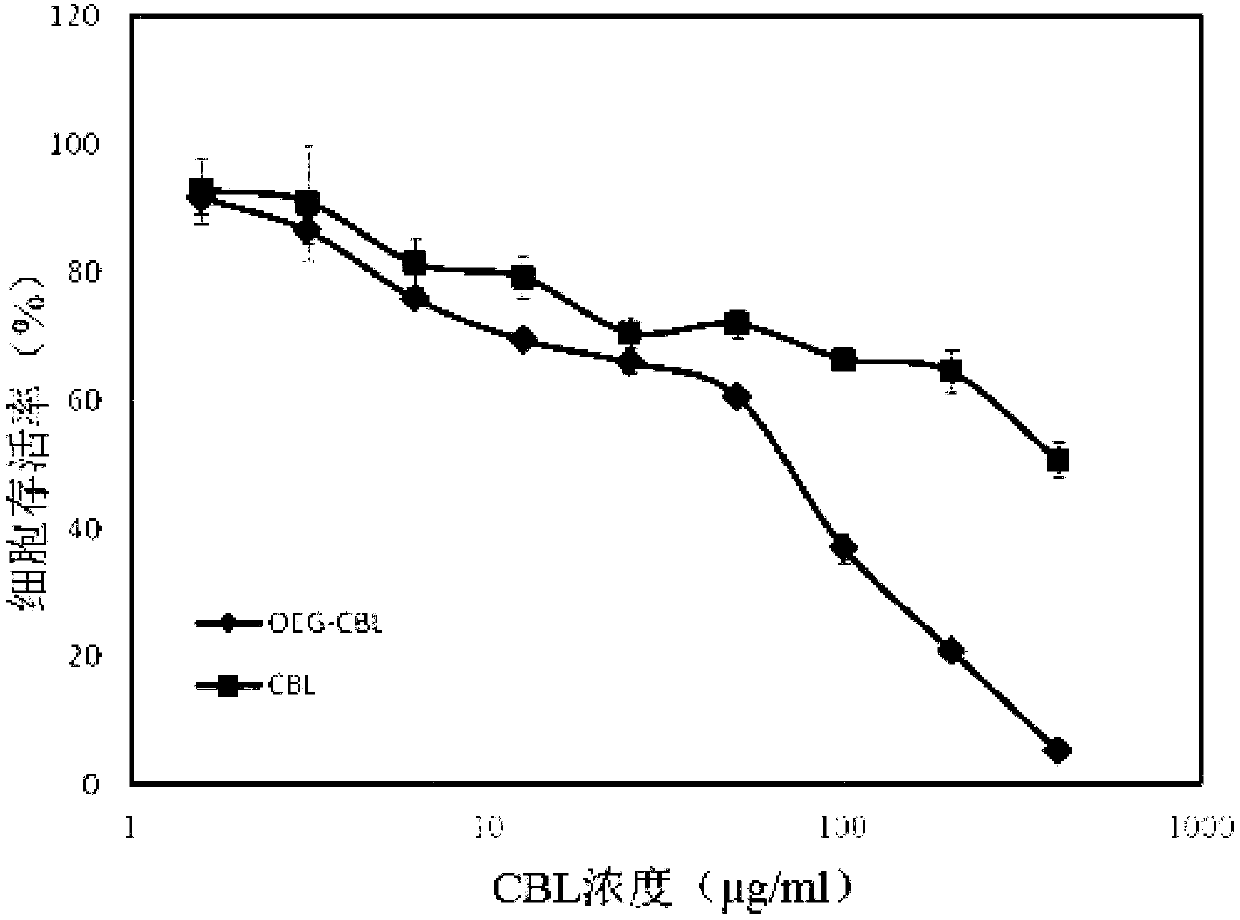
Oligo (ethylene glycol) modified chlorambucil nanomedicine and preparation method thereof
A technology of chlorambucil and polyethylene glycol, which is applied in the directions of drug combination, pharmaceutical formulation, and non-active ingredient medical preparations, etc., can solve the problems of biocompatibility, poor druggability, and low drug load, etc. , to achieve the effect of high drug loading rate, strong druggability and high cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
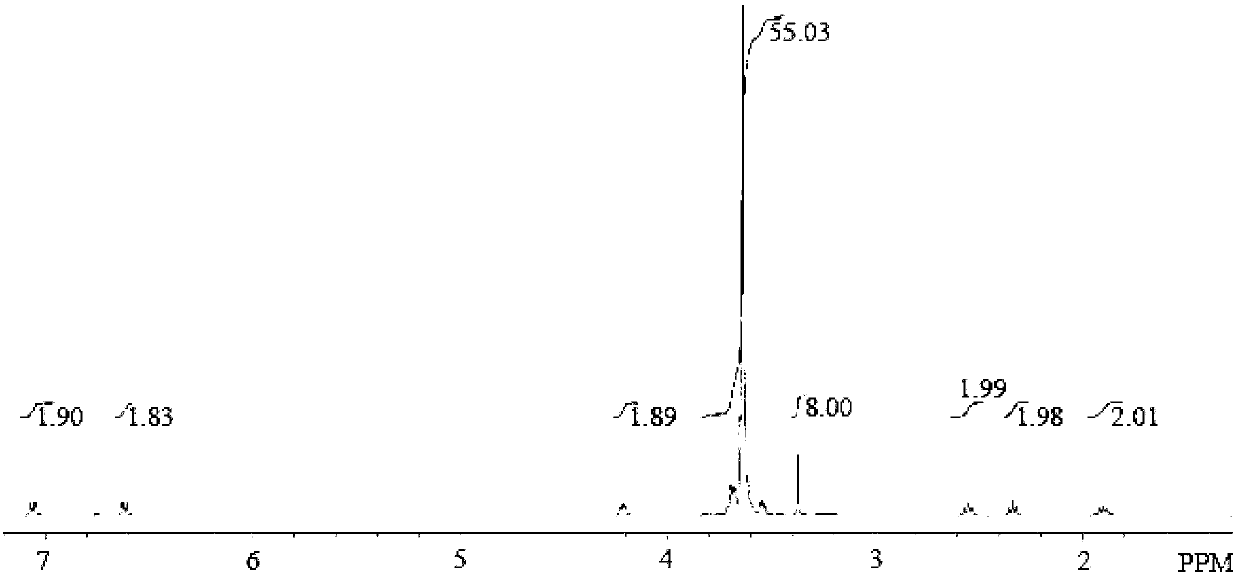
[0028] The preparation of embodiment 1 OEG-CBL
[0029]CBL (1.04 g, 3.42 mmol) was dissolved in 10 mL of dry pyridine, then EDC·HCl (0.6563 g, 3.42 mmol) and OEG (0.9437 g, 1.71 mmol, number average molecular weight 552) were added to the pyridine solution, under argon Under gas protection, react at room temperature at 30°C for 48h. After the reaction, pour the reaction liquid into 100mL n-hexane for precipitation, centrifuge to take the precipitate, dissolve it with an appropriate amount of chloroform and then precipitate it with n-hexane, repeat the precipitation twice, collect the precipitate, and pass through a 100-200 mesh coarse-pore column chromatography silica gel column Purification, the eluent is ethyl acetate, and a mixed solvent of dichloromethane and methanol with a volume ratio of 1:1, first rinsed with ethyl acetate until there is no excess CBL, and then replaced with a mixed solvent of dichloromethane and methanol 1:1 Washing yields the product OEG-CBL.
[00...
Embodiment 2
[0031] Example 2 Preparation of OEG-CBL
[0032] CBL (1.018 g, 3.35 mmol) was dissolved in 10 mL of dry pyridine, then EDC·HCl (0.6428 g, 3.35 mmol) and OEG (0.6235 g, 2.07 mmol, number average molecular weight 300) were added to the pyridine solution under argon Under gas protection, react at room temperature at 20°C for 48h. After the reaction, pour the reaction liquid into 100mL n-hexane for precipitation, centrifuge to take the precipitate, dissolve it with an appropriate amount of chloroform and then precipitate it with n-hexane, repeat the precipitation twice, collect the precipitate, and pass through a 100-200 mesh coarse-pore column chromatography silica gel column Purification, the eluent is ethyl acetate, and a mixed solvent of dichloromethane and methanol with a volume ratio of 1:1, first rinsed with ethyl acetate until there is no excess CBL, and then replaced with a mixed solvent of dichloromethane and methanol 1:1 The product was obtained by washing, and the obt...
Embodiment 3
[0033] Example 3 Preparation of OEG-CBL
[0034] CBL (0.4665 g, 1.534 mmol) was dissolved in 10 mL of dry pyridine, then EDC·HCl (0.2929 g, 1.534 mmol) and OEG (1.023 g, 1.023 mmol, number average molecular weight of 1000) were added to the pyridine solution under argon Under gas protection, react at room temperature at 18°C for 48h. After the reaction, pour the reaction liquid into 100mL n-hexane for precipitation, centrifuge to take the precipitate, dissolve it with an appropriate amount of chloroform and then precipitate it with n-hexane, repeat the precipitation 3 times, collect the precipitate, and pass through a 100-200 mesh coarse-pore column chromatography silica gel column Purification, the eluent is ethyl acetate, and a mixed solvent of dichloromethane and methanol with a volume ratio of 1:1, first rinsed with ethyl acetate until there is no excess CBL, and then replaced with a mixed solvent of dichloromethane and methanol 1:1 The product was obtained by washing, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com