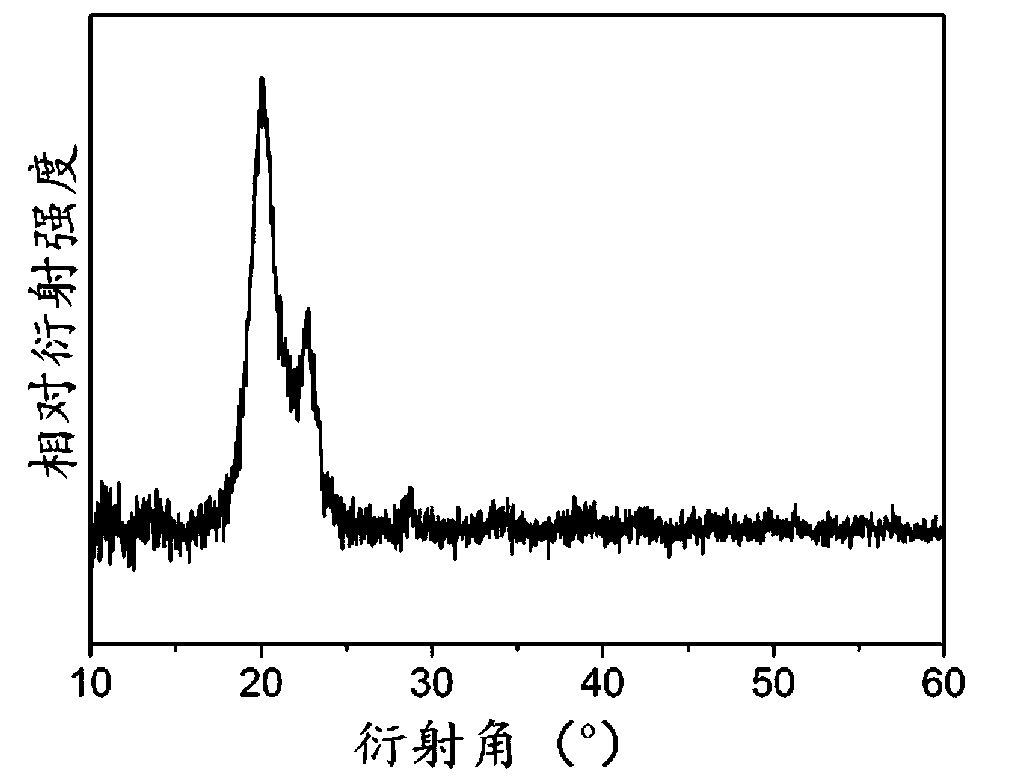
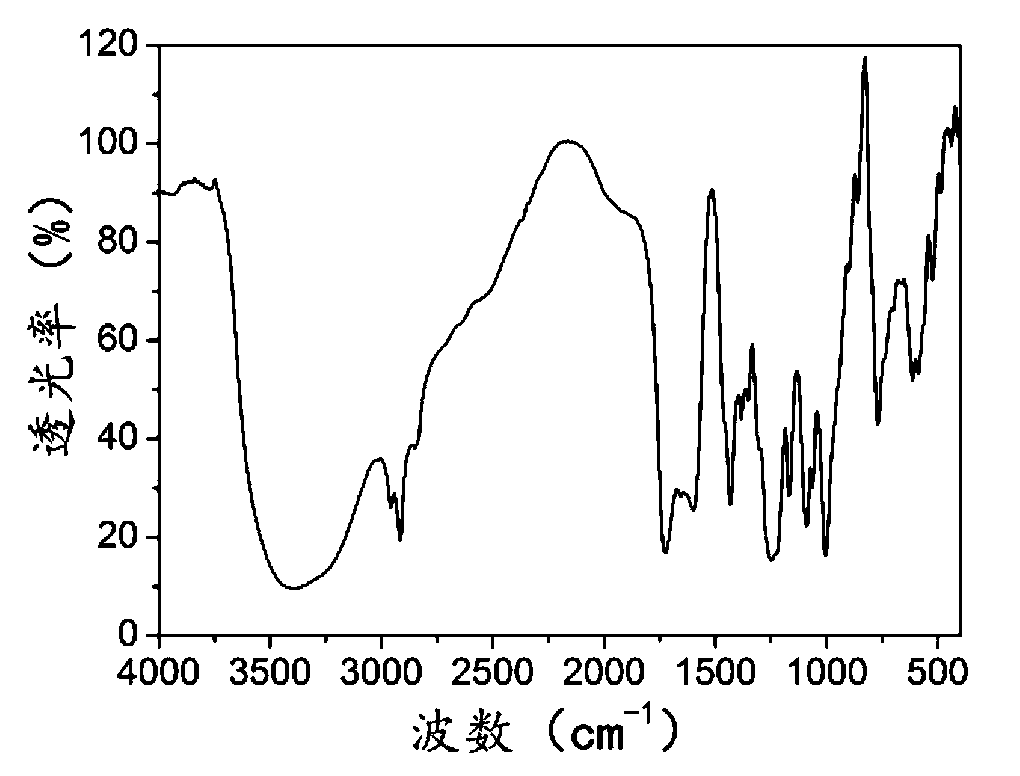
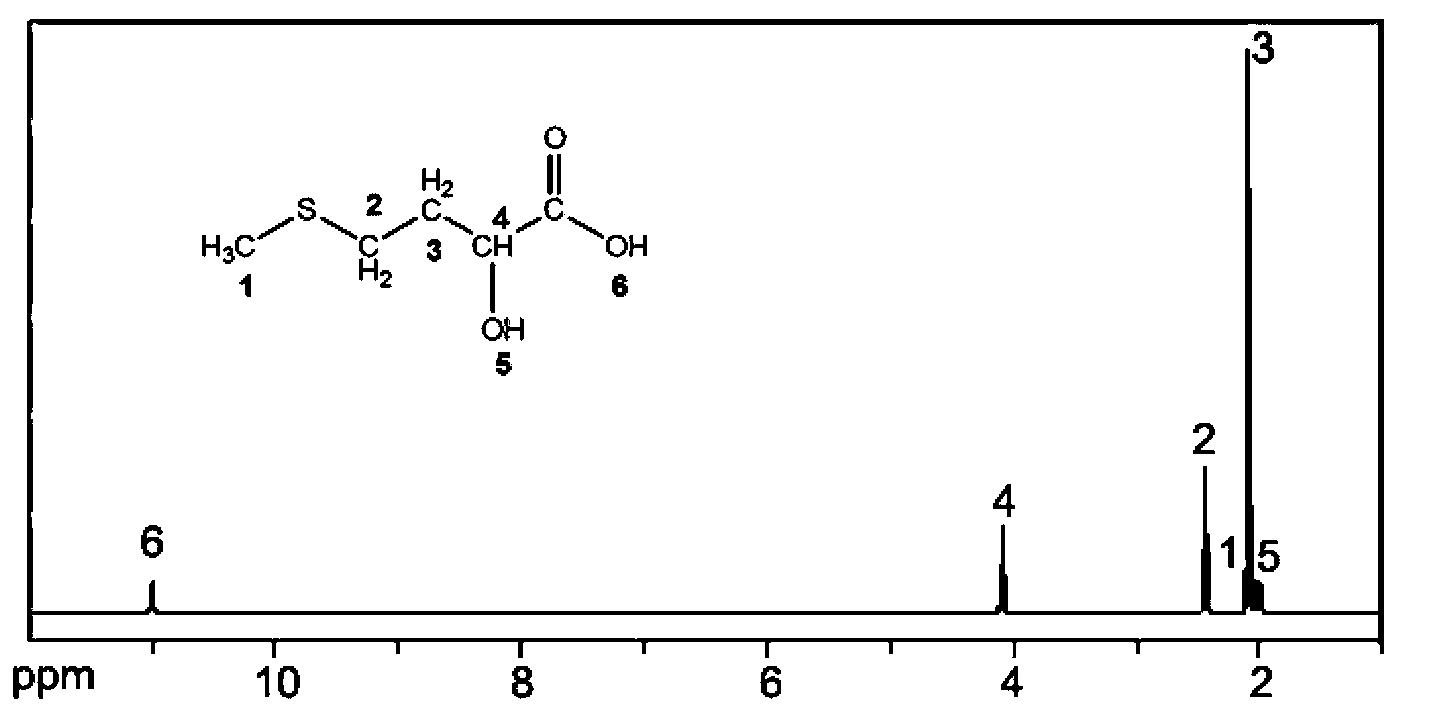
Method for preparing solid 2-hydroxy-4-(methylthio) butanoic acid
A technology of methylthiobutyric acid and hydroxymethionine, which is used in the production of food, feed additives and related fine chemicals, can solve the problems of high price, storage, transportation, and inconvenient use, and achieve the effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A preparation method of solid 2-hydroxyl-4-methylthiobutyric acid, comprising the steps of:
[0034] a. Add 3.7g of calcium hydroxide, 17.1g of hydroxymethionine, and 60mL of ethanol into a 100mL three-necked flask, and under stirring, heat the three-necked flask to 85°C, stir and reflux for 1 hour; Standby), drying to obtain calcium hydroxymethionine;
[0035] b. Add 3.38g of calcium hydroxymethionine prepared in step a, 1.0g of concentrated sulfuric acid and 49mL of ethyl acetate into a 100mL three-neck flask, stir and react at room temperature for 2 hours; separate the mixture from solid to liquid, the precipitate is calcium sulfate, collect Liquid, made hydroxymethionine solution;
[0036] c. Distill the hydroxymethionine solution prepared in step b in a water bath with a vacuum of 0.06MPa and 40°C under reduced pressure, and dry the white or light yellow solid precipitated in the solution to constant weight at 60°C to obtain solid 2 -Hydroxy-4-methylthiobutyric a...
Embodiment 2
[0042] A preparation method of solid 2-hydroxyl-4-methylthiobutyric acid, comprising the steps of:
[0043] a. Add 5g of calcium carbonate, 17.1g of hydroxymethionine, and 60mL of methanol into a 250mL three-neck flask, and under stirring, heat the three-necked flask to 85°C, stir and reflux for 1 hour; the product is filtered by suction and washed with alcohol (recover the filtrate for later use) , drying to obtain calcium hydroxymethionine;
[0044] b. Add 3.38g of calcium hydroxymethionine prepared in step a, 1.0g of concentrated sulfuric acid and 60mL of methanol into a 100mL three-neck flask, stir and react at room temperature for 0.5h; separate the mixture from solid to liquid, the precipitate is calcium sulfate, and collect the liquid , to obtain a hydroxymethionine solution;
[0045] c. The hydroxymethionine solution prepared in step b is distilled under reduced pressure in a water bath with a vacuum degree of 0.095MPa and 70°C, and the white or light yellow solid pre...
Embodiment 3
[0048] A preparation method of solid 2-hydroxyl-4-methylthiobutyric acid, comprising the steps of:
[0049] a. Add 7.67g of barium oxide, 17.1g of hydroxymethionine, and 116mL of ethanol into a 250mL three-necked flask, and under stirring, heat the three-necked flask to 90°C, stir and reflux for 6 hours; ), drying to obtain barium hydroxymethionate;
[0050] b. Add 4.36g of barium hydroxymethionine prepared in step a, 1.0g of concentrated sulfuric acid and 70mL of ethanol into a 100mL three-neck flask, stir and react at 50°C for 6h; separate the mixture from solid to liquid, the precipitate is barium sulfate, and collect the liquid , to obtain a hydroxymethionine solution;
[0051] c. The hydroxymethionine solution prepared in step b is distilled under reduced pressure in a water bath with a vacuum degree of 0.08MPa and 50°C, and the white or light yellow solid precipitated in the solution is dried to constant weight at 80°C to obtain solid 2- Hydroxy-4-methylthiobutyric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com