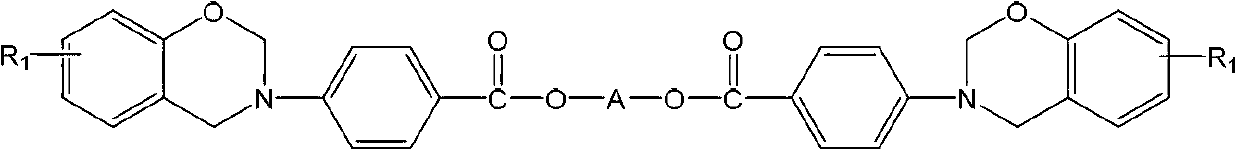
Ester-group-containing diamine type fluorenyl benzoxazine
A technology of ester-based diamine type and fluorenylbenzene, which is applied in the field of organic polymer materials, can solve the problems of limited application and high brittleness of polybenzoxazine resin, and achieve the effects of shortening reaction time, improving toughness and processing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 4.64g (0.01mol) 9,9-bis(4-aminobenzoic acid methyl) fluorene, 1.9g (0.02mol) phenol, 1.2 g (0.04mol) paraformaldehyde and 60mL xylene. Heat to 150°C and reflux for 6 hours. After the reaction, cool to room temperature, add ethanol, wash the precipitate several times, filter, and vacuum dry to obtain white powder 9,9-bis(4-aminobenzoic acid methyl)fluorene - Phenylbenzoxazine monomer (BAPMFp), yield 72.3%.
[0026] H NMR test results (500M, CDCl 3 , ppm): 6.61~7.92 (m, 24H, Ar-H), 5.40 (d, 4H, O-CH 2 -N), 4.71 (d, 4H, Ar-CH 2 -N), 4.66 (d, 4H, O-CH 2 -C); infrared spectrum test results (KBr, cm □1 ):2888 (stretching vibration of methylene), 1708 (stretching vibration of ester carbonyl), 1606 and 1455 (skeleton vibration of benzene ring), 1322 (swinging vibration of methylene on oxazine ring), 1101 and 1227 (C-O-C symmetry and asymmetric stretching vibration), 1182 (C-N-C symmetric and asymmetric stretching vibration), 954 (C-H out-of-plane bending vibration of ...
Embodiment 2
[0029] Except that the raw material phenol was changed to m-cresol, and the mass was 2.16g, other conditions were the same as in Example 1, and finally light yellow 9,9-bis(4-aminobenzoic acid methyl)fluorene-m-cresolyl benzoxazine was obtained Monomer (BAPMF-m-mp), yield 67.8%.
[0030] H NMR test results (500M, CDCl 3 , ppm): 6.65~7.93 (m, 22H, Ar-H), 5.42 (d, 4H, O-CH 2-N), 4.74 (d, 4H, Ar-CH 2 -N), 4.66 (d, 4H, O-CH2-C), 2.26 (d, 6H, -CH 3 ); infrared spectrum test results (KBr, cm -1 ): 2884, 1709, 1607, 1455, 1325, 1227, 1101, 1183 and 956.
[0031] Curing and testing conditions are the same as in Example 1, polybenzoxazine resin (poly(BAPMF-m-mp)), T g : 166,T 5 :379,Y C : 33%.
Embodiment 3
[0033] Except that the raw material phenol is used instead of p-cresol, and the reaction time is 15h, other conditions are the same as in Example 2, and finally obtain light yellow 9,9-bis(4-aminobenzoic acid methyl) fluorene-p-phenol benzoxazine mono body (BAPMF-p-mp), the yield was 61.2%.
[0034] H NMR test results (500M, CDCl 3 , ppm): 6.65~7.93 (m, 22H, Ar-H), 5.42 (d, 4H, O-CH 2 -N), 4.74 (d, 4H, Ar-CH 2 -N), 4.67 (d, 4H, O―CH2―C), 2.24 (d, 6H, -CH 3 ); infrared spectrum test results (KBr, cm -1 ): 2897, 1708, 1609, 1455, 1323, 1226, 1099, 1182 and 954.
[0035] Curing and testing conditions are the same as in Example 1, polybenzoxazine resin (poly(BAPMF-p-mp)), T g : 155,T 5 : 355, Y C : 31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com