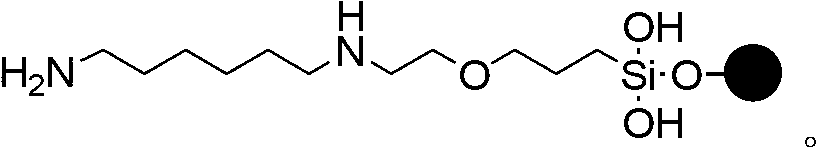
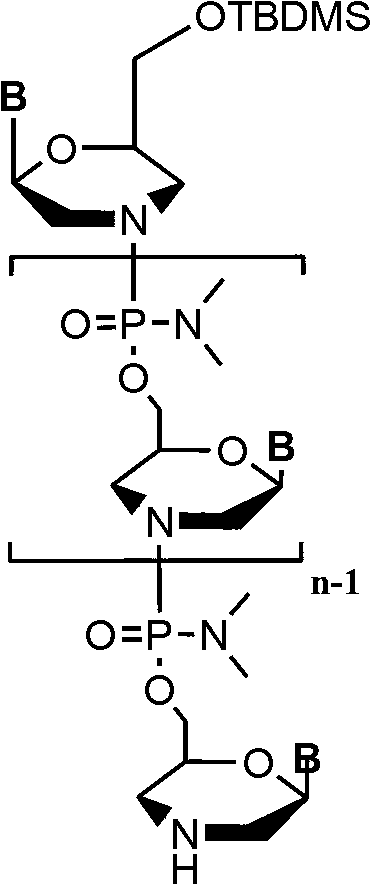
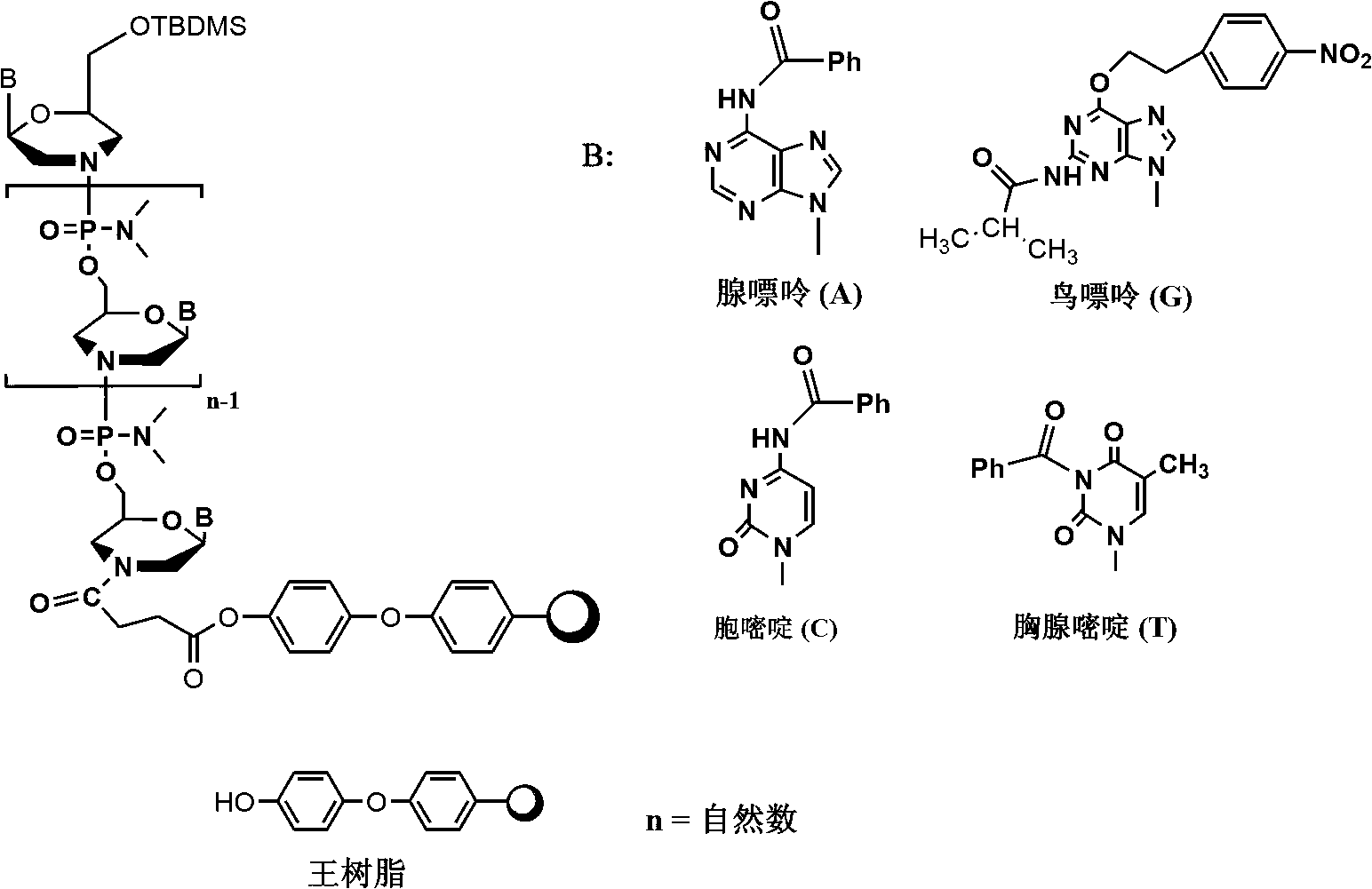
Phosphorodiamidate morpholino oligomer synthetized by solid phase and method thereof
A technology of phosphorodiamidate morpholine and solid-phase synthesis, which is applied in the fields of chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve problems such as high synthesis cost and expensive resin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: the synthesis of compound PMO (A-T):
[0066] Taking bases A and T as an example, the synthesis route of PMO (A-T) is:
[0067]
[0068] The feed ratio (molar ratio) of T-5 and tert-butyldimethylchlorosilane is 1 / 1.3, dissolved in dichloromethane. Add imidazole under stirring at room temperature, and the molar ratio of the added amount of imidazole to the morpholino derivative is 3 / 1. After reacting for 5 hours, the reactant was washed with water, dried with anhydrous sodium sulfate, and the crude product T-6 was obtained as a colorless oil after removing the solvent.
[0069] Second step reaction:
[0070]
[0071] T-6 was dissolved in trifluoroacetic acid containing acetic acid, the volume ratio of acetic acid and trifluoroacetic acid was 1 / 50, stirred for 4 hours, and then the solvent was removed to obtain T-7.
[0072] The third step reaction:
[0073]
[0074] T-7 was dissolved in water and dioxane (volume ratio: 1 / 1), and sodium carbonat...
Embodiment 2
[0090] Embodiment 2: the synthesis of compound PMO (C-A-T):
[0091] synthetic route:
[0092]
[0093] First step response:
[0094]
[0095] Compounds C-8 and R-4 (molar feeding ratio: 9 / 1) were dissolved in triethylamine dichloromethane solution (concentration: 50 mg / ml) and placed in a solid-phase synthesis tube. R-5 was obtained after overnight reaction and washed with copious amounts of dichloromethane.
[0096] Second step reaction:
[0097]
[0098] Add tetrabutylammonium fluoride tetrahydrofuran solution (solution concentration: 100 mg / ml) into the solid-phase synthesis tube equipped with R-5. Then the solid-phase synthesis tube was placed on a shaker to react for 4 hours, and the solid-phase resin was washed with tetrahydrofuran and dichloromethane to obtain R-6.
[0099] Tenth step reaction:
[0100]
[0101] Under the condition of ammonia water, R-4 was cut off from the solid phase support of Wang resin to obtain PMO (C-A-T). The amino group on t...
Embodiment 3
[0102] Embodiment 3: the synthesis of compound PMO (G-C-A-T):
[0103]
[0104] First step response:
[0105]
[0106] Compounds G-8 and R-6 (molar feed ratio: 2 / 1) were dissolved in triethylamine dichloromethane solution (concentration: 50 mg / ml) and placed in a solid-phase synthesis tube. R-7 was obtained after overnight reaction and washed with copious amounts of dichloromethane.
[0107] Second step reaction:
[0108]
[0109] Add tetrabutylammonium fluoride tetrahydrofuran solution (solution concentration: 100 mg / ml) into the solid-phase synthesis tube equipped with R-7. Then the solid-phase synthesis tube was placed on a shaker to react for 4 hours, and the solid-phase resin was washed with tetrahydrofuran and dichloromethane to obtain R-8.
[0110] The third step reaction:
[0111]
[0112] Under the condition of ammonia water, R-8 was cut off from the solid phase support of Wang resin to obtain PMO (G-C-A-T). The amino group on the terminal morpholine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com