Preparation method of triblock polymers with suspension nanometer zero-valent irons
A technology of nano-zero-valent iron and polymers, applied in chemical instruments and methods, and other chemical processes, to achieve the effects of improving reactivity and suspension performance, increasing yield, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
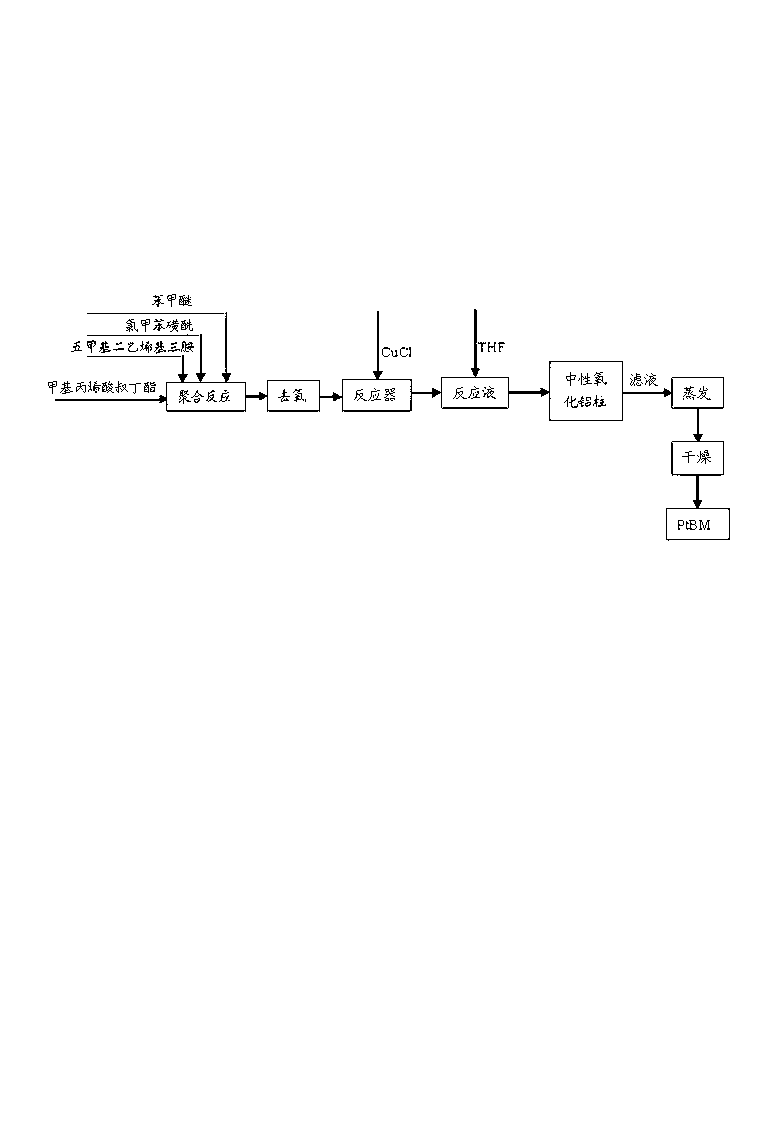
[0032] Embodiment 1: as Figure 6Shown, the preparation method of polymethacrylic acid-polymethylmethacrylate-polystyrenesulfonic acid triblock polymer (PMAA-PMMA-PSS) comprises the following steps:
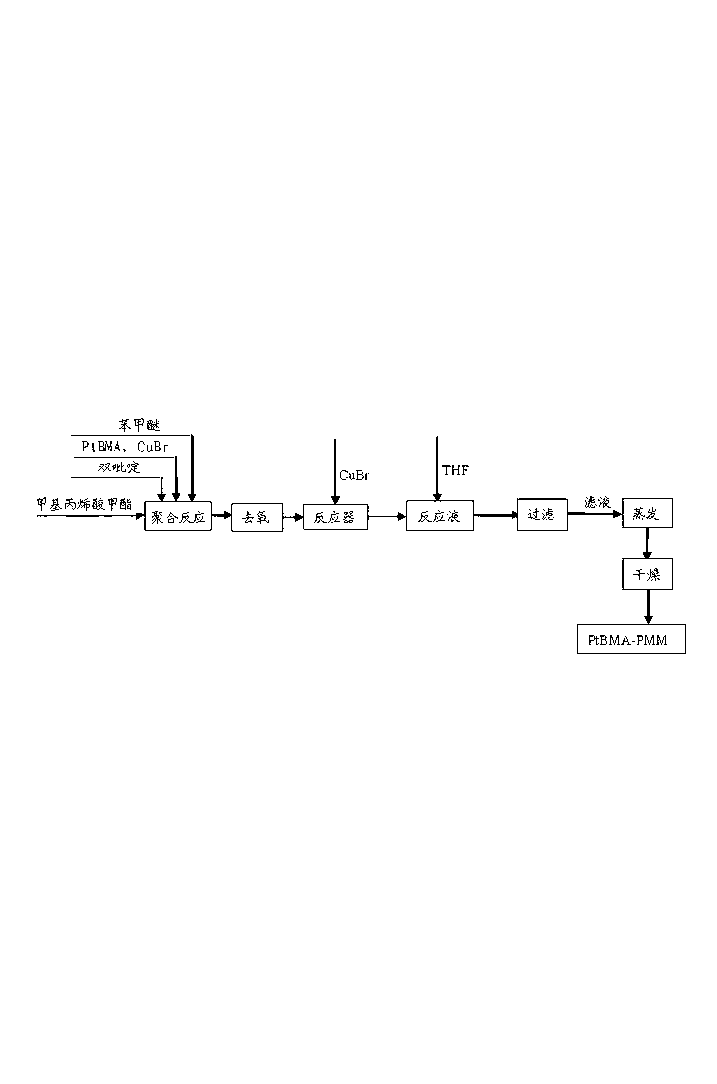
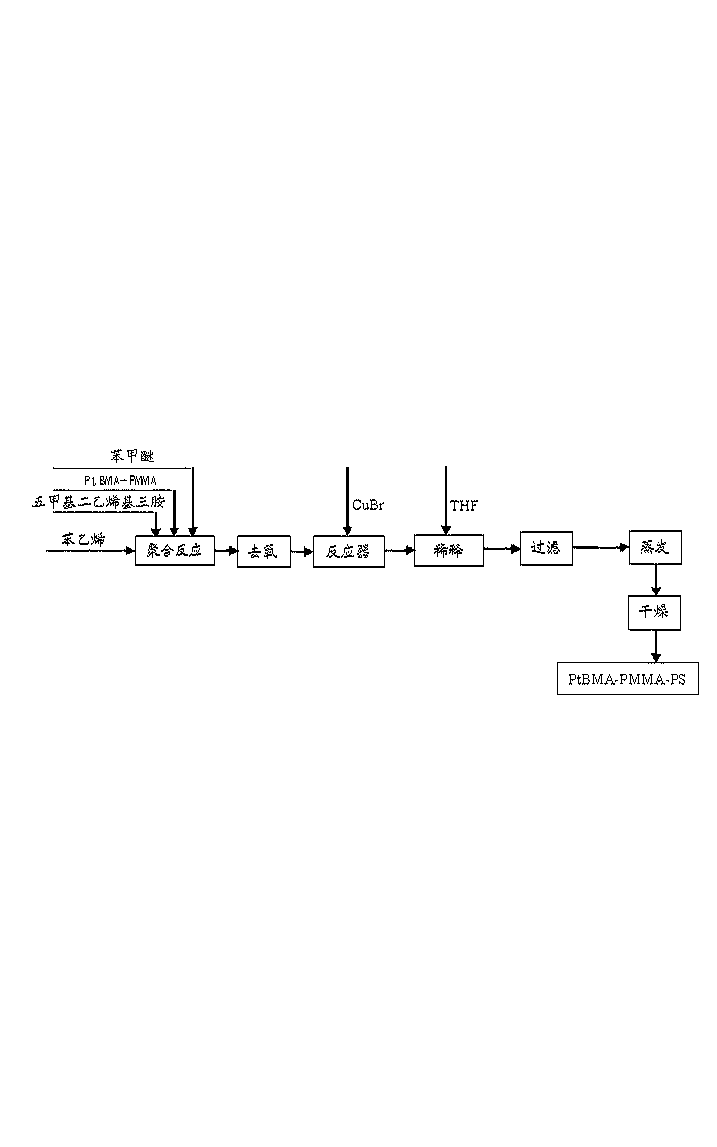
[0033] A. Synthesis of PtBMA-PMMA-PSt: Add 4.88g of anisole, 0.19g of chlorotoluenesulfonyl, 0.35g of pentamethyldivinyltriamine and 4.26g of tert-butyl methacrylate to the reactor and stir to dissolve , and then freeze with liquid nitrogen, vacuumize, and blow nitrogen to deoxygenate (repeat three times), then add 0.11g CuCl under nitrogen protection, and then keep the reaction system at 60°C and continue to react under nitrogen blowing. After 12 hours of reaction, 1.61 g of anisole and 1.51 g of methyl methacrylate were added, and the reaction system was kept at 30° C. and the reaction was carried out under continuous nitrogen flow. After reacting for 12 hours, 34.21 g of anisole and 31.25 g of styrene were added, and the reaction system was kept at 80° C. under continuous nit...
Embodiment 2
[0036] A. Synthesis of PtBMA-PMMA-PSt: Add 9.51g of anisole, 0.19g of chlorotoluenesulfonyl, 0.51g of pentamethyldivinyltriamine and 8.53g of tert-butyl methacrylate to the reactor and stir to dissolve , and then freeze with liquid nitrogen, vacuumize, and blow nitrogen to remove oxygen (repeat three times), then add 0.15g CuCl under nitrogen protection, and then keep the reaction system at 50°C and continue to react under nitrogen blowing. After reacting for 10 hours, 4.51 g of anisole and 4.01 g of methyl methacrylate were added, and the reaction system was kept at 250° C. and the reaction was carried out under continuous nitrogen flow. After reacting for 10 hours, 65.51 g of anisole and 62.49 g of styrene were added, and the reaction system was kept at 85° C. and the reaction was carried out under continuous nitrogen flow. After reacting for 60 hours, the reaction mixture was diluted with 1L tetrahydrofuran (THF), then the diluted solution was filtered through a neutral alu...
Embodiment 3
[0039] A. Synthesis of PtBMA-PMMA-PSt: Add 13.89g of anisole, 0.38g of chlorotoluenesulfonyl, 1.04g of pentamethyldivinyltriamine and 12.79g of tert-butyl methacrylate to the reactor and stir to dissolve , and then freeze with liquid nitrogen, vacuumize, and blow nitrogen to deoxygenate (repeat three times), then add 0.29g CuCl under nitrogen protection, and then keep the reaction system at 40°C and continue to react under nitrogen blowing. After reacting for 11 hours, 4.89 g of anisole and 4.01 g of methyl methacrylate were added, and the reaction system was kept at 35° C. and the reaction was carried out under continuous nitrogen flow. After reacting for 11 hours, 98.88 g of anisole and 93.74 g of styrene were added, and the reaction system was kept at 75° C. and the reaction was carried out under continuous nitrogen flow. After 40 hours of reaction, the reaction mixture was diluted with 1.5L tetrahydrofuran (THF), then the diluted solution was filtered in a neutral alumina ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com