PH response four-arm star block copolymer and preparation method and application thereof
A technology of block copolymers and polymers, which can be used in pharmaceutical combinations, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., and can solve the problems of insufficient stability and controlled release performance of multi-arm star block copolymers. , to achieve the effect of improving pH response sensitivity and release efficiency, improving controlled release performance, and controlling drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
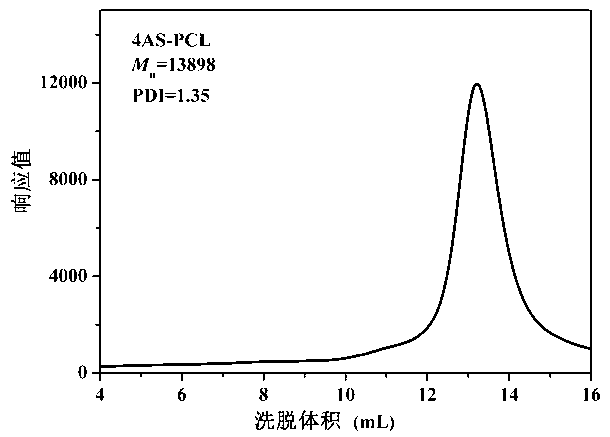
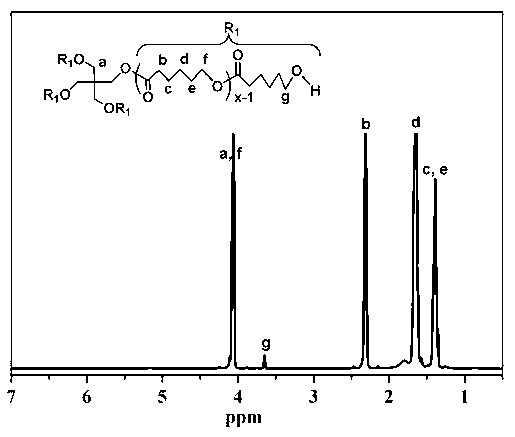
[0069] (1) Synthesis of polymers grafted with hydrophobic groups: put a stirrer and 0.136g of pentaerythritol in a reaction bottle, seal it and vacuumize it - blow argon three times, then use a syringe to pour 20mL of solvent toluene, 12g of monomer e-CL and 0.012g Sn(Oct) 2 Add it into the reaction flask, use liquid nitrogen for three times of freezing-pumping-heating cycle, and stir the reaction in an oil bath at 120°C under the protection of argon for 36h. After the reaction is completed, cool to room temperature, evaporate toluene under reduced pressure, and add 50mL THF Diluted, then precipitated with 300mL 0°C methanol / water (1:1 volume ratio), dried in vacuum at 45°C for 24h, and obtained a white powder, which was the polymer 4AS-PCl grafted with hydrophobic groups, with a yield of 91%. M n =13898, PDI=1.35;
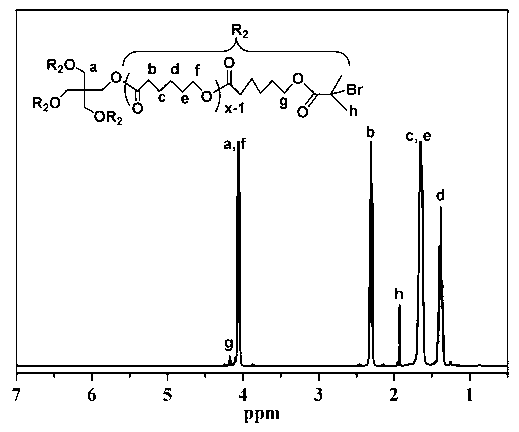
[0070] (2) Synthesis of macromolecular initiator: Take a dry three-necked flask, add 12g 4AS-PCl and 150mL THF, seal it and pass argon for 10 minutes, inject 2....
Embodiment 2
[0078] (1) Synthesis of 4AS-PCl: Put a stirrer and 0.136g of pentaerythritol in the reaction flask, seal it and pump it in vacuum-argon three times, then add 20mL of solvent toluene, 10g of monomer e-CL and 0.020g of Sn( Oct) 2 , after three cycles of freezing-pumping-heating with liquid nitrogen, the reaction was stirred in an oil bath at 140°C under the protection of argon for 24 hours. After the reaction was completed, it was cooled to room temperature, the toluene was distilled off under reduced pressure, diluted with 50mL THF, and then used 300mL of methanol / water (1:1 volume ratio) at 0°C was precipitated and dried in vacuum at 45°C for 24h to obtain a white powder which was the product 4AS-PCl with a yield of 92%. M n =11036, PDI=1.60;
[0079] (2) Synthesis of 4AS-PCL-Br: Take a dry three-necked flask, add 10g 4AS-PCl and 150mL THF, seal it and pass argon for 10min, then add 1.62g TEA after sealing, cool to 0°C with an ice-water bath, then add 3.68g 2-Bromoisobutyry...
Embodiment 3
[0082] (1) Synthesis of 4AS-PCl: Put a stirrer and 0.136g of pentaerythritol in the reaction bottle, seal it and pump it with argon for three times, then add 20mL of solvent toluene, 18g of monomer e-CL and 0.018g of Sn( Oct) 2 , after three cycles of freezing-pumping-heating with liquid nitrogen, stir the reaction in an oil bath at 130°C under the protection of argon for 36h, after the completion of the reaction, cool to room temperature, evaporate toluene under reduced pressure, add 50mL THF to dilute, and then use 300mL of methanol / water (1:1 volume ratio) at 0°C was precipitated and dried in vacuum at 45°C for 24h to obtain a white powder which was the product 4AS-PCl with a yield of 86%. M n =18025, PDI=1.59;
[0083] (2) Synthesis of 4AS-PCL-Br: Take a dry 250mL three-neck flask, add 18g 4AS-PCl and 150mL THF, seal it and pass argon for 10min, add 2.02g TEA after sealing, cool to 0°C with an ice-water bath, and then add 4.6g of 2-bromoisobutyryl bromide was first reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical micelle concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com