Method for making 1,1-difluoroethylene polymer
A technology of vinylidene fluoride and a manufacturing method, applied in 1 field, can solve the problems of complicated polymerization operation, increased equipment cost burden, temperature rise above critical temperature, etc., and achieves the effects of high bulk density and easy scale coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0061] Hereinafter, examples are given to further describe the present invention in detail, but the present invention is not limited to the examples.
[0062] The physical properties of the vinylidene fluoride polymer powders obtained in Examples and Comparative Examples were measured by the following methods.
[0063] 〔Inherent viscosity〕
[0064] 4 g of 1,1-vinylidene fluoride polymer powder was added to 1 liter of N,N-dimethylformamide, and a solution was prepared for dissolving at 80° C. for 8 hours. This solution was kept at 30° C., the logarithmic viscosity was measured with an Ubbelohde viscometer, and the inherent viscosity was obtained from the following formula. Logarithmic viscosity [η] = ln (η rel ) / C
[0065] Among them, η rel Indicates the number of falling seconds of the sample solution / the number of falling seconds of the solvent, and C represents the concentration of the sample solution (0.4g / dl).
[0066] 〔SEM observation〕
[0067] The 1,1-vinylidene fl...
Embodiment 1
[0083] 1024g of ion-exchanged water, 0.4g of methylcellulose, 0.2g of sorbitan trioleate (span85, referring to the following formula (1)), 400g of 1,1- Ethylene difluoride, 0.6 g of diisopropyl peroxydicarbonate (IPP), and 1.8 g of ethyl acetate were subjected to suspension polymerization at 26° C. for 31 hours. The polymerization yield of the suspension polymerization was 89%.
[0084] After the polymerization, the polymer slurry was heat-treated at 95°C for 30 minutes, dehydrated, washed with water, and dried at 80°C for 20 hours to obtain vinylidene fluoride polymer powder (1).
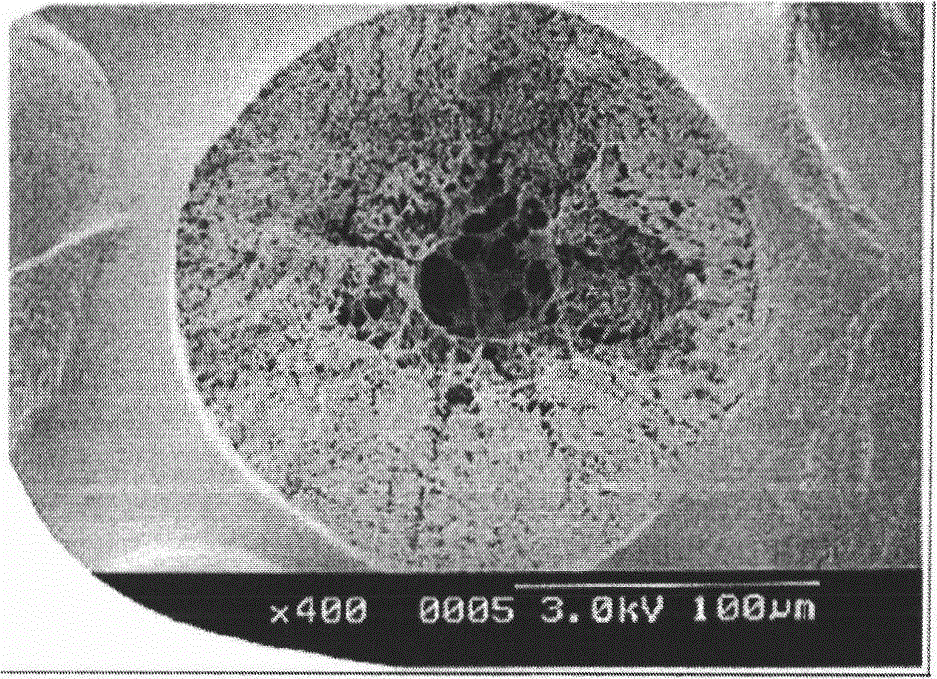
[0085] The obtained 1,1-difluoroethylene polymer powder (1) had an inherent viscosity of 3.1 dl / g, an average particle diameter of 150 μm, and a bulk density of 0.46 g / cm 3 . In addition, the SEM photograph of the cross section of the vinylidene fluoride polymer powder (1) is shown in figure 2 .
[0086]
Embodiment 2
[0088] 1024g of ion-exchanged water, 0.2g of methyl cellulose, 0.4g of sorbitan trioleate (span85), 400g of 1,1-difluoroethylene, 0.6g of peroxide were put into an autoclave with an inner volume of 2 liters. Diisopropyl dicarbonate (IPP), 1.8 g of ethyl acetate, and suspension polymerization at 26° C. for 79 hours. The polymerization yield of the suspension polymerization was 89%.
[0089] After the polymerization, the polymer slurry was heat-treated at 95°C for 30 minutes, dehydrated, washed with water, and dried at 80°C for 20 hours to obtain vinylidene fluoride polymer powder (2).
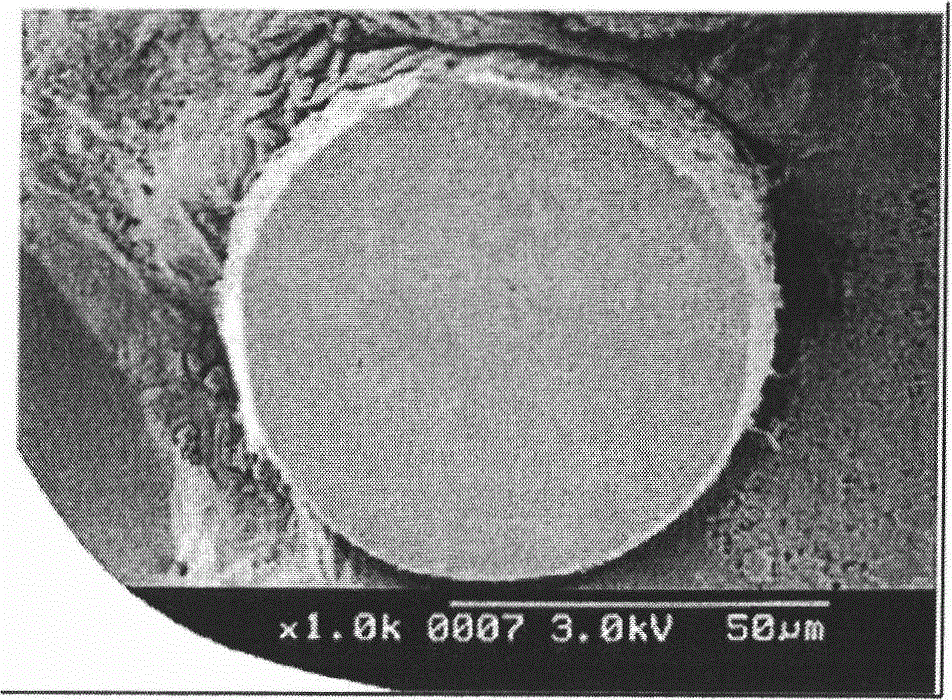
[0090] The obtained 1,1-difluoroethylene polymer powder (2) had an inherent viscosity of 3.1 dl / g, an average particle diameter of 220 μm, and a bulk density of 0.53 g / cm 3 . In addition, the SEM photograph of the cross section of 1,1-vinylidene fluoride polymer powder (2) is shown in image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inherent viscosity | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com