Manganese ion-activated red long-afterglow luminescent material and preparation method thereof
A technology of long afterglow luminescence and manganese ions, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., to achieve the effects of good performance, simple preparation method, and stable chemical properties of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
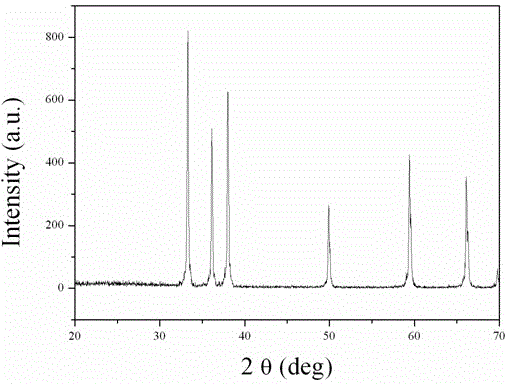
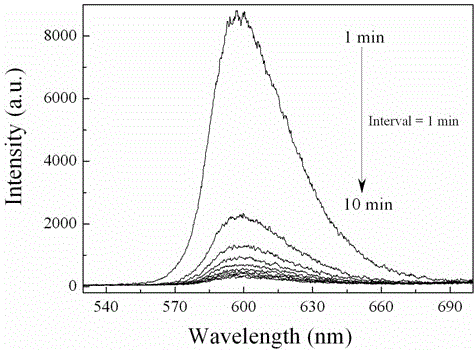
[0020] Weigh 1.6231 g of analytically pure aluminum nitride (AlN), and analyze pure manganese carbonate (MnCO 3 )0.0460 g, where Mn 2+ The doping concentration of ions is 0.1% (molar percentage). After fully wet grinding in an agate mortar, put it into a boron nitride crucible, compact it, cover it, put the crucible into a high-temperature furnace, heat it to 1700°C, and keep the temperature constant for 1-3 hours. Cool to room temperature with the furnace to obtain a near-white powder. Through XRD identification, the product is aluminum nitride pure phase, figure 1 Shown is the XRD pattern of the obtained sample. The emission spectrum includes a broad band from 570nm to 700nm. After being irradiated with 254 nm ultraviolet light for 1 minute, the luminescent material exhibits obvious red long afterglow emission, and the afterglow time is about 0.5 hours.
Embodiment 2
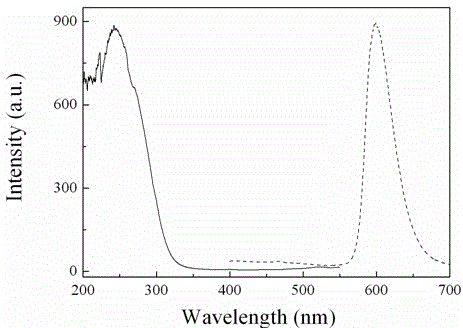
[0022] Weigh 1.5985 g of analytically pure aluminum nitride (AlN), and analyze pure manganese carbonate (MnCO 3 ) 0.1150 g, where Mn 2+ The doping concentration of ions is 2.5% (molar percentage). After fully wet grinding in an agate mortar, put it into a boron nitride crucible, compact it, cover it, put the crucible into a high-temperature furnace, heat it to 1750°C, and keep the temperature constant for 3 hours. Cool to room temperature with the furnace to obtain a near-white powder. According to XRD identification, the product is a pure phase of aluminum nitride, the emission spectrum includes a wide band from 570 nm to 700 nm, and the half-peak width is 43 nm. After being irradiated with 254 nm ultraviolet light for 1 minute, the luminescent material exhibits strong red light with long afterglow emission, and the afterglow time is about 2 hours. figure 2 are the excitation and emission spectra of the sample. Under the irradiation of ultraviolet light, the measured lum...
Embodiment 3
[0024] Weigh 1.4756 g of analytically pure aluminum nitride (AlN), and analyze pure manganese carbonate (MnCO 3 ) 0.4598 g, where Mn 2+ The doping concentration of ions is 10%. After fully wet grinding in an agate mortar, put it into a boron nitride crucible, compact it, cover it, put the crucible into a high-temperature furnace, heat it to 1700°C, and keep the temperature constant for 3 hours. The body color of the obtained powder sample after cooling with the furnace is off-white. According to XRD identification, the main phase of the product is aluminum nitride, and contains a small amount of Al 2 o 3 , the emission spectrum includes a broad band from 570 nm to 700 nm. After being irradiated with 254 nm ultraviolet light for 1 minute, the luminescent material exhibits obvious long afterglow emission of red light, and the afterglow time is about 20 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com